Chonnam Med J.
2008 Dec;44(3):162-168. 10.4068/cmj.2008.44.3.162.
Protective Effects of K6PC-5, A Sphingosine Kinase Activator, Against 1-methyl-4-phenylpyridinium-induced Dopaminergic Neuronal Cell Death
- Affiliations
-
- 1Department of Physiology, Chonnam National University Medical School, Gwangju, Korea.
- 2Chonnam National University Research Institute of Medical Sciences, Gwangju, Korea. jhsjeong@hanmail.net
- 3Department of Familial Medicine, Yeosu Chonnam Hospital, Yeosu, Korea.
- KMID: 2172296
- DOI: http://doi.org/10.4068/cmj.2008.44.3.162
Abstract
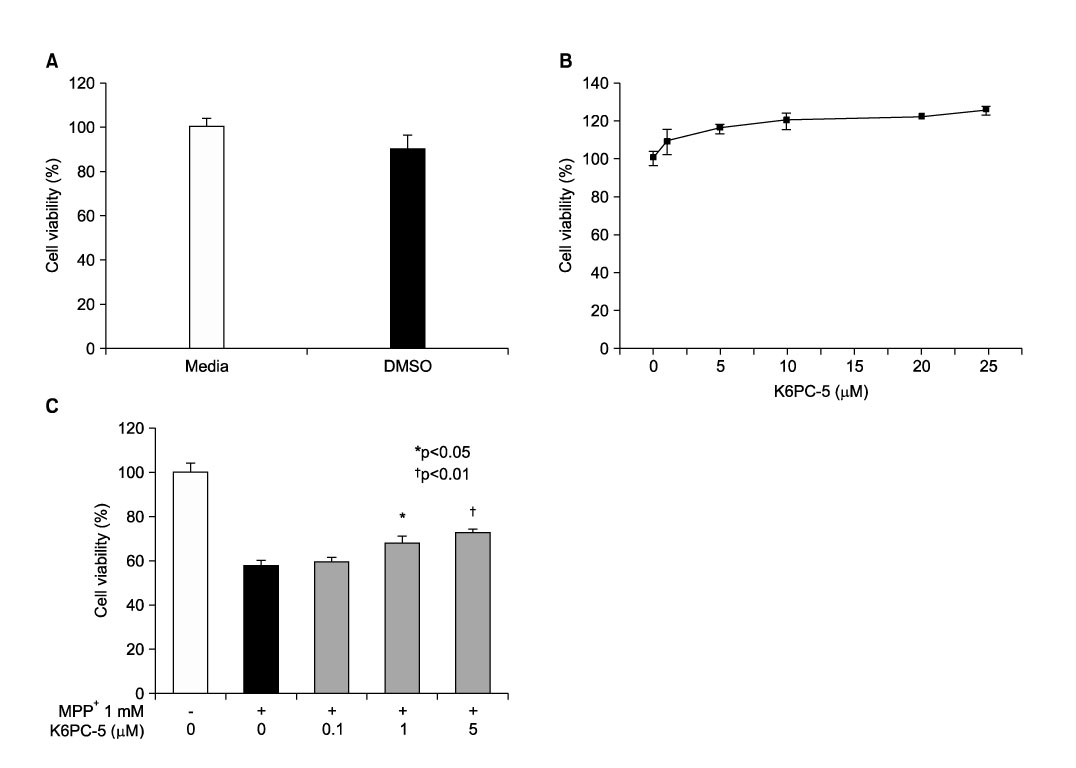
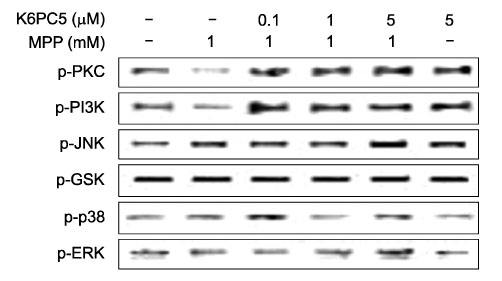
- Sphingosine-1-phosphate (S1P), a bioactive sphingolipid metaolite, regulates multiple cellular responses such as Ca2+ signaling, cellular growth and survival, and differentiation. Sphingosine kinase (SphK) is a key enzyme in modulating the levels of S1P and is emerging as an important regulating enzyme. Here we have investigated whether K6PC-5, a newly synthesized SphK activator, plays a neuroprotective role by activating cell survival systems such as protein kinase C, phosphatidylinositol-3-kinase (PI3K), and acting on the anti-apoptotic and the pro-apoptotic genes in SN4741 dopaminergic cells. 1-methyl-4-phenylpyridinium is a neurotoxin that selectively inhibits the mitochondrial functions of dopaminergic neurons in the substantia nigra pars compacta. In the present study, we found that MPP+ induced a decrease in SN4741 mouse dopaminergic cell viability. K6PC-5 restored the reduced phospho-PKC and phospho-PI3K activities caused by MPP+ toxicity. In addition, gene expression analysis revealed that K6PC-5 prevented both the MPP+ -induced expression of the pro-apoptotic gene mRNA, Bax, and the decrease of the anti-apoptotic gene mRNA, bcl-w. These results suggest that the neuroprotective mechanism of K6PC-5 against MPP+ -induced apoptotic cell death includes stimulation of PKC and PI3K, and modulation of cell survival and death genes.
Keyword
MeSH Terms
-
1-Methyl-4-phenylpyridinium
Amides
Animals
Cell Death
Cell Survival
Dopamine Agents
Dopaminergic Neurons
Gene Expression
Lysophospholipids
Mice
Parkinson Disease
Phosphotransferases
Phosphotransferases (Alcohol Group Acceptor)
Protein Kinase C
RNA, Messenger
Sphingosine
Substantia Nigra
1-Methyl-4-phenylpyridinium
Amides
Dopamine Agents
Lysophospholipids
Phosphotransferases
Phosphotransferases (Alcohol Group Acceptor)
Protein Kinase C
RNA, Messenger
Sphingosine
Figure
Reference
-
1. Gerlach M, Ben-Shachar D, Riederer P, Youdim MB. Altered brain metabolism of iron as a cause of neurodegenerative diseases. J Neurochem. 1994. 63:793–807.
Article2. Jenner P, Olanow CW. Oxidative stress and the pathogenesis of Parkinson's disease. Neurology. 1996. 47:6 Suppl 3. S161–S170.
Article3. Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003. 53:Suppl 3. S26–S38.
Article4. Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003. 39:889–909.5. Noh HG, Jang SJ, Park SJ, Kim SH, Jeong HS, Park JS. Protective effects of epigallocatechin-3-gallate against 1-methyl-4-phenylpyridinium-induced dopaminergic neuronal cell death. Chonnam Med J. 2007. 43:73–79.6. Krishnamurthi R, Stott S, Maingay M, Faull RL, McCarthy D, Gluckman P, et al. N-terminal tripeptide of IGF-1 improves functional deficits after 6-OHDA lesion in rats. Neuroreport. 2004. 15:1601–1604.
Article7. Schober A. Classic toxin-induced animal models of Parkinson's disease: 6-OHDA and MPTP. Cell Tissue Res. 2004. 318:215–224.
Article8. Ryu EJ, Angelastro JM, Greene LA. Analysis of gene expression changes in a cellular model of Parkinson disease. Neurobiol Dis. 2005. 18:54–74.
Article9. Huwiler A, Kolter T, Pfeilschifter J, Sandhoff K. Physiology and pathophysiology of sphingolipid metabolism and signaling. Biochim Biophys Acta. 2000. 1485:63–99.
Article10. Zhang YH, Vasko MR, Nicol GD. Intracellular sphingosine 1-phosphate mediates the increased excitability produced by nerve growth factor in rat sensory neurons. J Physiol. 2006. 575(Pt 1):101–113.
Article11. Liu H, Chakravarty D, Maceyka M, Milstien S, Spiegel S. Sphingosine kinases: a novel family of lipid kinases. Prog Nucleic Acid Res Mol Biol. 2002. 71:493–511.
Article12. Shu X, Wu W, Mosteller RD, Broek D. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Mol Cell Biol. 2002. 22:7758–7768.
Article13. Edsall LC, Cuvillier O, Twitty S, Spiegel S, Milstien S. Sphingosine kinase expression regulates apoptosis and caspase activation in PC12 cells. J Neurochem. 2001. 76:1573–1584.
Article14. Taha TA, Hannun YA, Obeid LM. Sphingosine kinase: biochemical and cellular regulation and role in disease. J Biochem Mol Biol. 2006. 39:113–131.
Article15. Olivera A, Kohama T, Edsall L, Nava V, Cuvillier O, Poulton S, et al. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol. 1999. 147:545–558.
Article16. Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003. 4:397–407.
Article17. Youm JK, Jo H, Hong JH, Shin DM, Kwon MJ, Jeong SK, et al. K6PC-5, a sphingosine kinase activator, induces anti-aging effects in intrinsically aged skin through intracellular Ca(2+) signaling. J Dermatol Sci. 2008. 51:89–102.
Article18. Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996. 381:800–803.
Article19. Xia P, Wang L, Moretti PA, Albanese N, Chai F, Pitson SM, et al. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-alpha signaling. J Biol Chem. 2002. 277:7996–8003.
Article20. Cordey M, Gundimeda U, Gopalakrishna R, Pike CJ. Estrogen activates protein kinase C in neurons: role in neuroprotection. J Neurochem. 2003. 84:1340–1348.
Article21. Levites Y, Amit T, Youdim MBH, Mandel S. Involvement of protein kinase C activation and cell survival/ cell cycle genes in green tea polyphenol (-)-epigallocatechin 3-gallate neuroprotective action. J Biol Chem. 2002. 277:30574–30580.
Article22. Gubina E, Rinaudo MS, Szallasi Z, Blumberg PM, Mufson RA. Overexpression of protein kinase C isoform epsilon but not delta in human interleukin-3-dependent cells suppresses apoptosis and induces bcl-2 expression. Blood. 1998. 91:823–829.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protective Effects of Epigallocatechin-3-gallate against 1-methyl-4-phenylpyridinium-induced Dopaminergic Neuronal Cell Death
- Role of Casein Kinase 2 in Parkinsonian Toxin 1-Methyl-4-phenylpyridinium-induced Cell Death
- Glucose Levels in Culture Medium Determine Cell Death Mode in MPP+-treated Dopaminergic Neuronal Cells
- Inhibitory Effect of Rotundarpene on Parkinsonian Neurotoxin 1-Methyl-4-Phenylpyridinium-Induced Apoptotic Cell Death
- Functional Role of Parkin against Oxidative Stress in Neural Cells