Endocrinol Metab.
2014 Mar;29(1):62-69. 10.3803/EnM.2014.29.1.62.
Functional Role of Parkin against Oxidative Stress in Neural Cells
- Affiliations
-
- 1Department of Biomedical Sciences, Korea University College of Medicine, Seoul, Korea. geumd@korea.ac.kr
- 2Department of Emergency Medical Technology, Kyungil University College of Nursing and Public Health, Gyeongsan, Korea. yhkim01@kiu.ac.kr
- KMID: 2169353
- DOI: http://doi.org/10.3803/EnM.2014.29.1.62
Abstract
- BACKGROUND
Parkinson disease (PD) is caused by selective cell death of dopaminergic neurons in the substantia nigra. An early onset form of PD, autosomal recessive juvenile parkinsonism has been associated with a mutation in the parkin gene. The function of parkin is known to remove misfolding proteins and protect cell death. We aimed to investigate the role of parkin against oxidative stress in neuronal cells.
METHODS
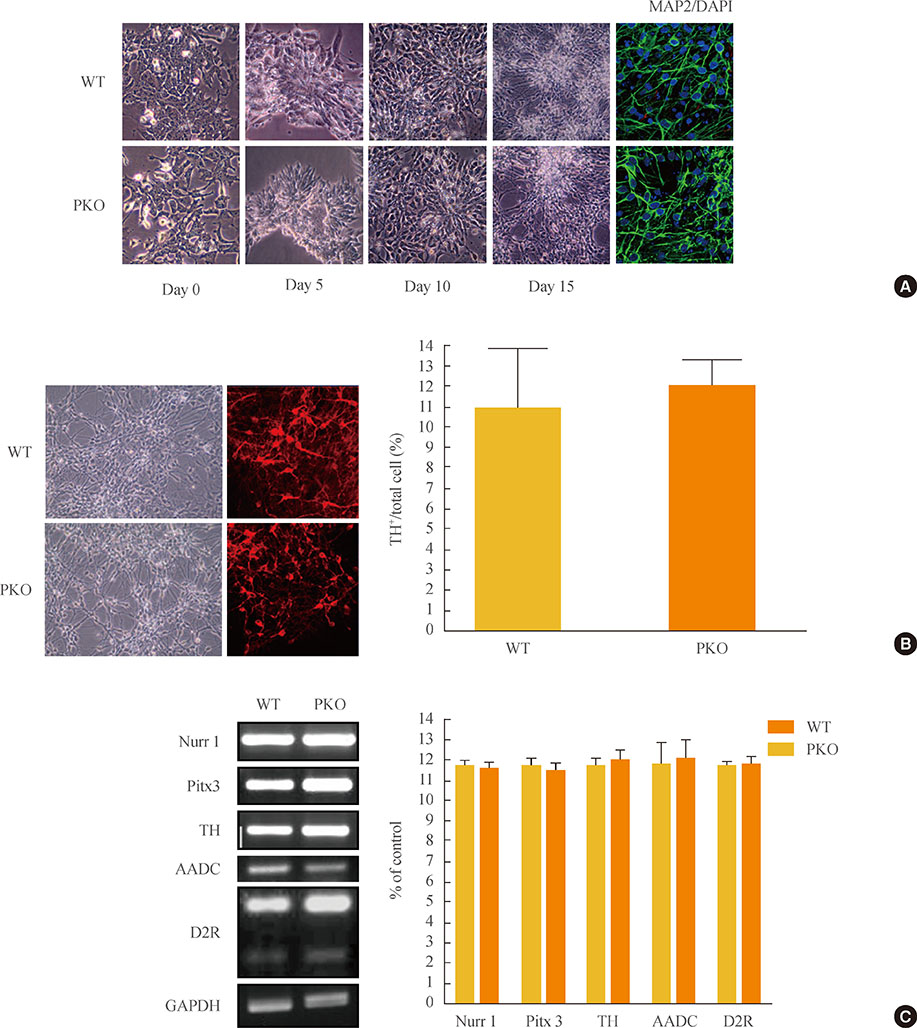
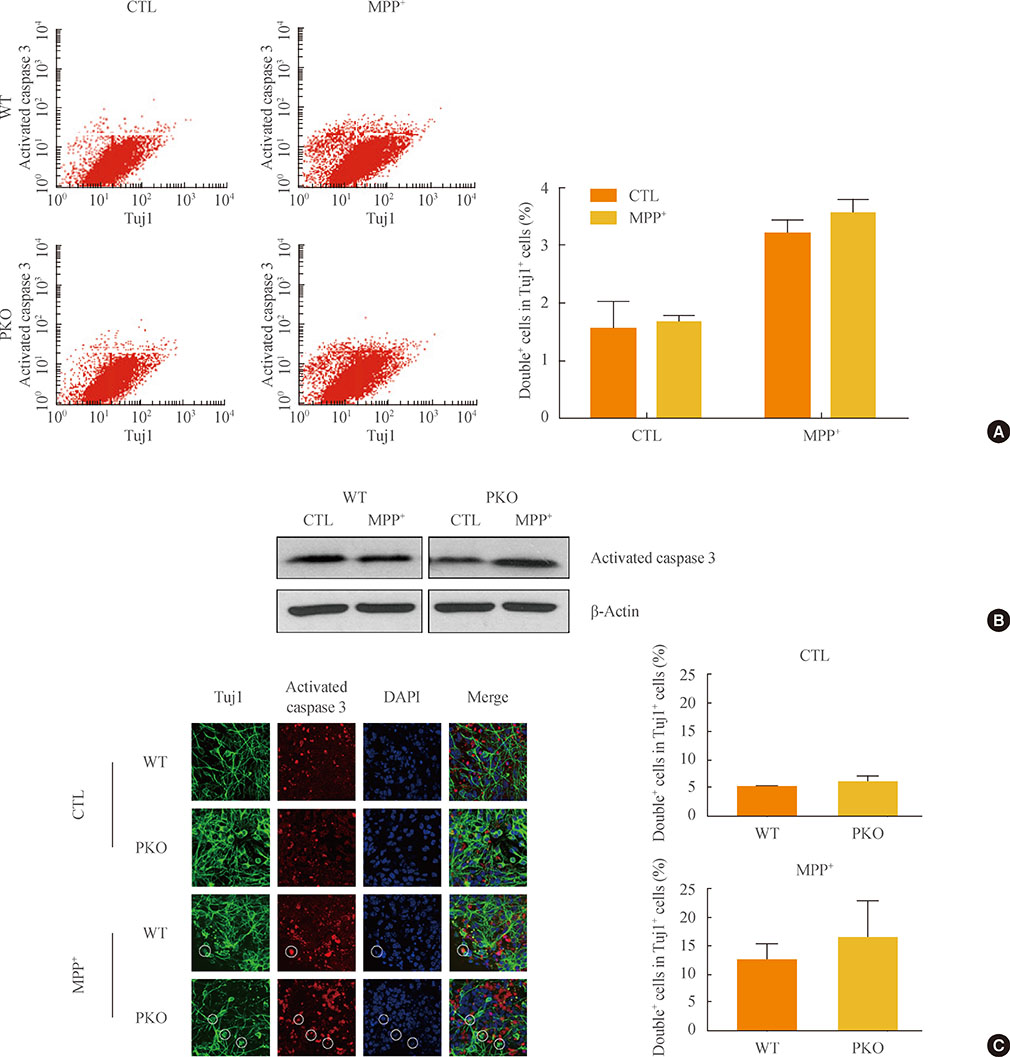
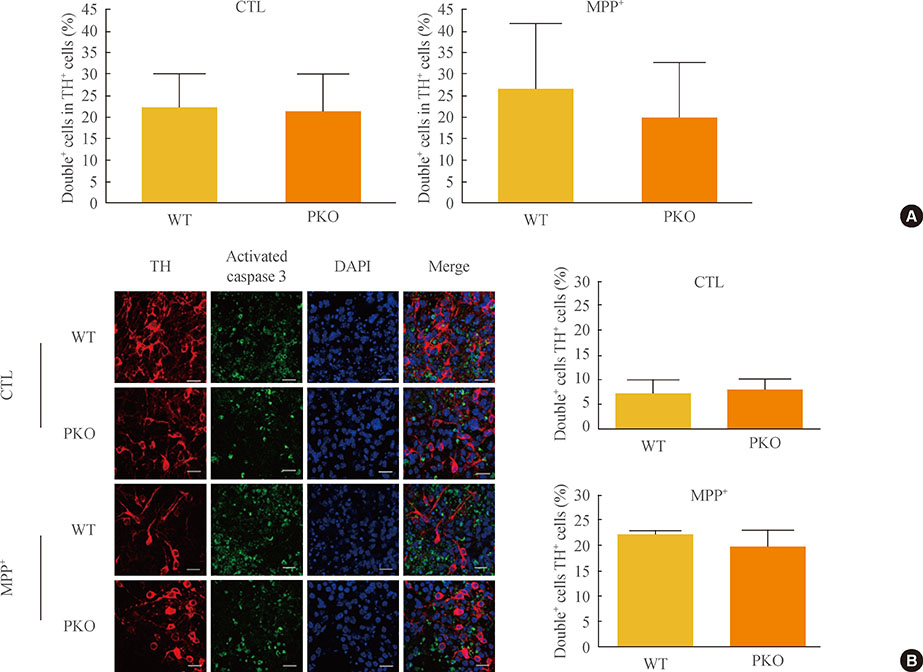
Parkin knockout embryonic stem cells (PKO ES cells) were differentiated into neurons by adherent monolayer culture method. Oxidative stress was induced by the treatment of 1-methyl-4-phenylpyridinium (MPP+) in neurons derived from wild type and PKO ES cells, and cell viability was examined by MTT assay. After exposure to MPP+, Tuj1-positive cell population was compared between PKO and wild type cells by fluorescence activated cell sorter (FACS) analysis. The activated caspase3 protein level was also measured by Western blot analysis, FACS and immunocytochemistry.
RESULTS
There was no difference in the efficiency of neuronal differentiation between wild type and PKO ES cells. After exposure to MPP+, no significant differences were found in cell viability and Tuj1-positive cell population between the two groups determined by MTT assay and FACS analysis, respectively. The activated caspase3 protein levels examined by Western blot analysis, FACS and immunocytochemistry were not changed in PKO cells compared with those of wild type cells after MPP+ treatment.
CONCLUSION
These results suggest that PKO neuronal cells including dopaminergic neurons are not sensitive to caspase3-dependent cell death pathway during the response against MPP+-induced oxidative stress.
MeSH Terms
Figure
Reference
-
1. Bossy-Wetzel E, Schwarzenbacher R, Lipton SA. Molecular pathways to neurodegeneration. Nat Med. 2004; 10:Suppl. S2–S9.2. Taylor JP, Hardy J, Fischbeck KH. Toxic proteins in neurodegenerative disease. Science. 2002; 296:1991–1995.3. Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, Jenner P, Halliwell B. Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem. 1997; 69:1196–1203.4. Arlt S, Beisiegel U, Kontush A. Lipid peroxidation in neurodegeneration: new insights into Alzheimer's disease. Curr Opin Lipidol. 2002; 13:289–294.5. Gabbita SP, Lovell MA, Markesbery WR. Increased nuclear DNA oxidation in the brain in Alzheimer's disease. J Neurochem. 1998; 71:2034–2040.6. Pedersen WA, Fu W, Keller JN, Markesbery WR, Appel S, Smith RG, Kasarskis E, Mattson MP. Protein modification by the lipid peroxidation product 4-hydroxynonenal in the spinal cords of amyotrophic lateral sclerosis patients. Ann Neurol. 1998; 44:819–824.7. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000; 408:239–247.8. Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, Minoshima S, Shimizu N, Iwai K, Chiba T, Tanaka K, Suzuki T. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000; 25:302–305.9. Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Wacker M, Klose J, Shen J. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem. 2004; 279:18614–18622.10. Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006; 441:1162–1166.11. Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, Chung J. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006; 441:1157–1161.12. Dodson MW, Guo M. Pink1, Parkin, DJ-1 and mitochondrial dysfunction in Parkinson's disease. Curr Opin Neurobiol. 2007; 17:331–337.13. Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003; 21:183–186.14. Darios F, Corti O, Lucking CB, Hampe C, Muriel MP, Abbas N, Gu WJ, Hirsch EC, Rooney T, Ruberg M, Brice A. Parkin prevents mitochondrial swelling and cytochrome c release in mitochondria-dependent cell death. Hum Mol Genet. 2003; 12:517–526.15. Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, Lam HA, Bhatnagar A, Meloni EG, Wu N, Ackerson LC, Klapstein GJ, Gajendiran M, Roth BL, Chesselet MF, Maidment NT, Levine MS, Shen J. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem. 2003; 278:43628–43635.16. Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983; 219:979–980.17. Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. Lancet. 1989; 1:1269.18. Dipasquale B, Marini AM, Youle RJ. Apoptosis and DNA degradation induced by 1-methyl-4-phenylpyridinium in neurons. Biochem Biophys Res Commun. 1991; 181:1442–1448.19. Du Y, Dodel RC, Bales KR, Jemmerson R, Hamilton-Byrd E, Paul SM. Involvement of a caspase-3-like cysteine protease in 1-methyl-4-phenylpyridinium-mediated apoptosis of cultured cerebellar granule neurons. J Neurochem. 1997; 69:1382–1388.20. Hartley A, Stone JM, Heron C, Cooper JM, Schapira AH. Complex I inhibitors induce dose-dependent apoptosis in PC12 cells: relevance to Parkinson's disease. J Neurochem. 1994; 63:1987–1990.21. Itano Y, Nomura Y. 1-methyl-4-phenyl-pyridinium ion (MPP+) causes DNA fragmentation and increases the Bcl-2 expression in human neuroblastoma, SH-SY5Y cells, through different mechanisms. Brain Res. 1995; 704:240–245.22. Thomas B, von Coelln R, Mandir AS, Trinkaus DB, Farah MH, Leong Lim K, Calingasan NY, Flint Beal M, Dawson VL, Dawson TM. MPTP and DSP-4 susceptibility of substantia nigra and locus coeruleus catecholaminergic neurons in mice is independent of parkin activity. Neurobiol Dis. 2007; 26:312–322.23. Fallon L, Belanger CM, Corera AT, Kontogiannea M, Regan-Klapisz E, Moreau F, Voortman J, Haber M, Rouleau G, Thorarinsdottir T, Brice A, van Bergen En Henegouwen PM, Fon EA. A regulated interaction with the UIM protein Eps15 implicates parkin in EGF receptor trafficking and PI(3)K-Akt signalling. Nat Cell Biol. 2006; 8:834–842.24. Henn IH, Bouman L, Schlehe JS, Schlierf A, Schramm JE, Wegener E, Nakaso K, Culmsee C, Berninger B, Krappmann D, Tatzelt J, Winklhofer KF. Parkin mediates neuroprotection through activation of IkappaB kinase/nuclear factor-kappaB signaling. J Neurosci. 2007; 27:1868–1878.25. Jiang H, Jiang Q, Feng J. Parkin increases dopamine uptake by enhancing the cell surface expression of dopamine transporter. J Biol Chem. 2004; 279:54380–54386.26. Jiang H, Ren Y, Zhao J, Feng J. Parkin protects human dopaminergic neuroblastoma cells against dopamine-induced apoptosis. Hum Mol Genet. 2004; 13:1745–1754.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epilepsy and Oxidative Stress
- Reactive Oxygen Species: Role in Pathophysiology, and Mechanism of Endogenous and Dietary Antioxidants during Oxidative Stress
- The Role of Oxidative Stress in the Pathogenesis of Asthma
- The role of oxidative stress and hypoxia in renal disease
- Effect of Hydrogen Peroxide-induced Oxidative Stress on the Senescence of Trabecular Meshwork Cells