J Bacteriol Virol.
2013 Jun;43(2):148-154. 10.4167/jbv.2013.43.2.148.
Current Understanding of HMGB1-mediated Autophagy
- Affiliations
-
- 1Department of Microbiology, Yonsei University College of Medicine, Seoul, Korea. jsshin6203@yuhs.ac
- 2Institute for Immunology and Immunological Diseases and Severance Biomedical Science Institute, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2168685
- DOI: http://doi.org/10.4167/jbv.2013.43.2.148
Abstract
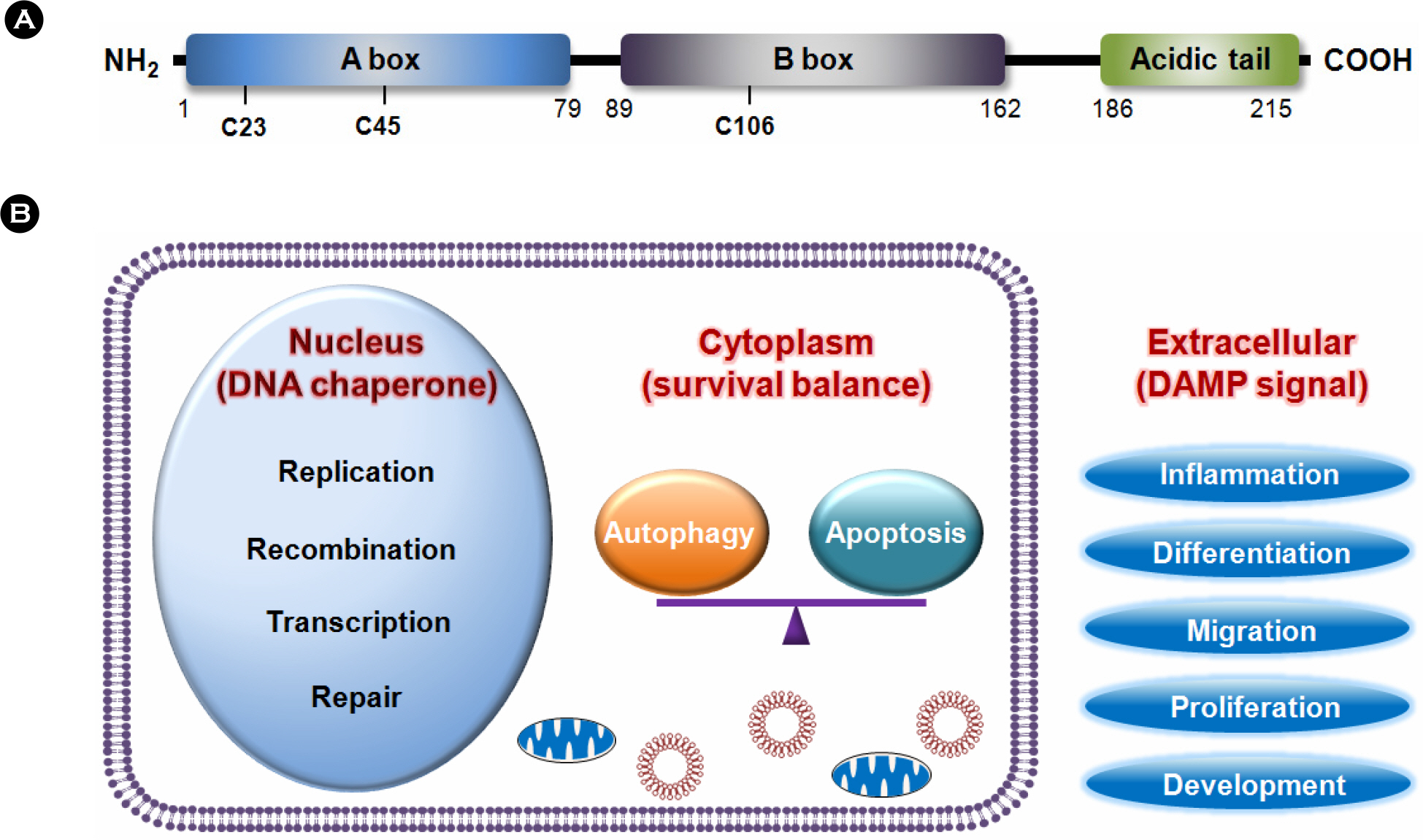
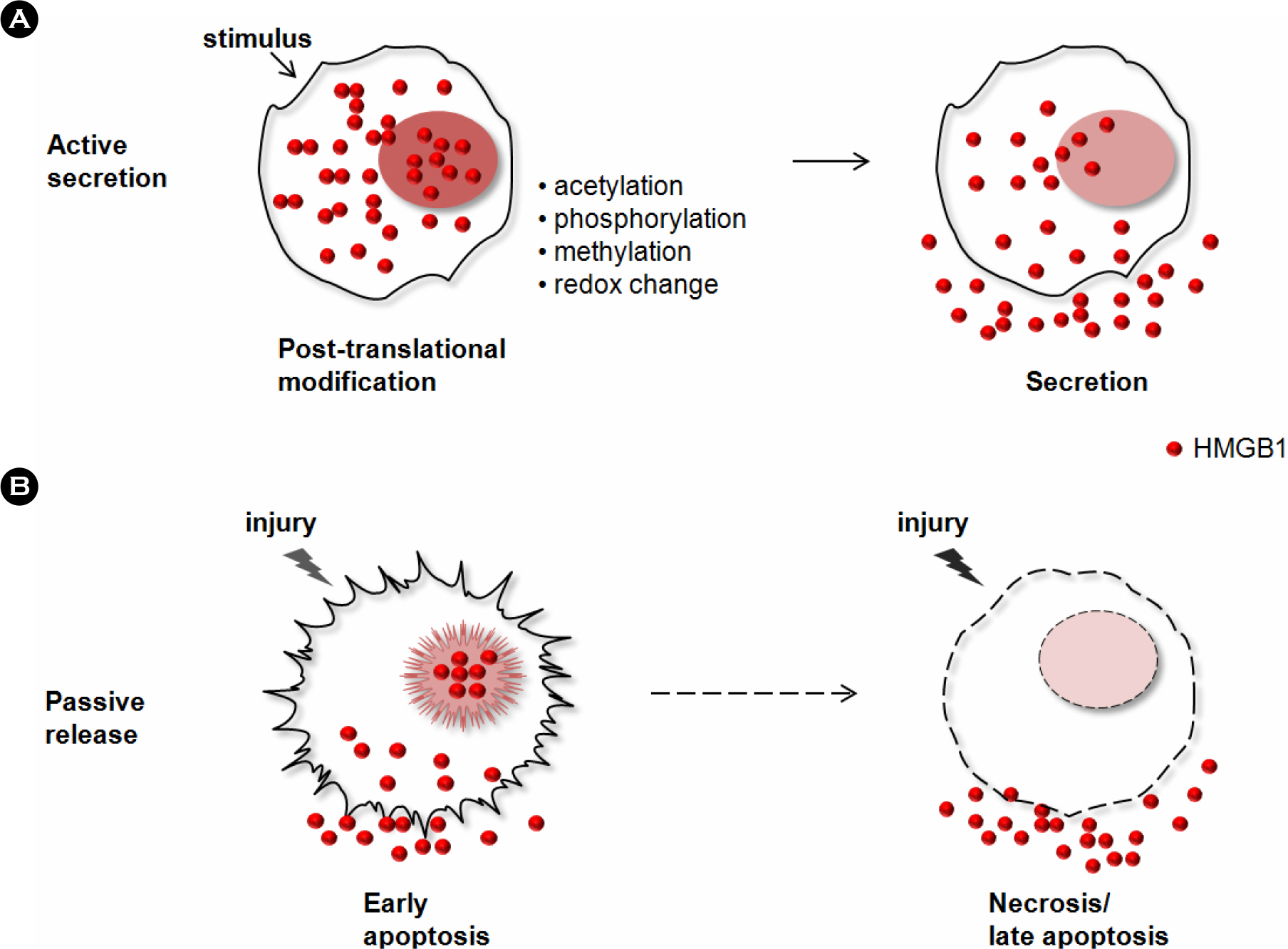
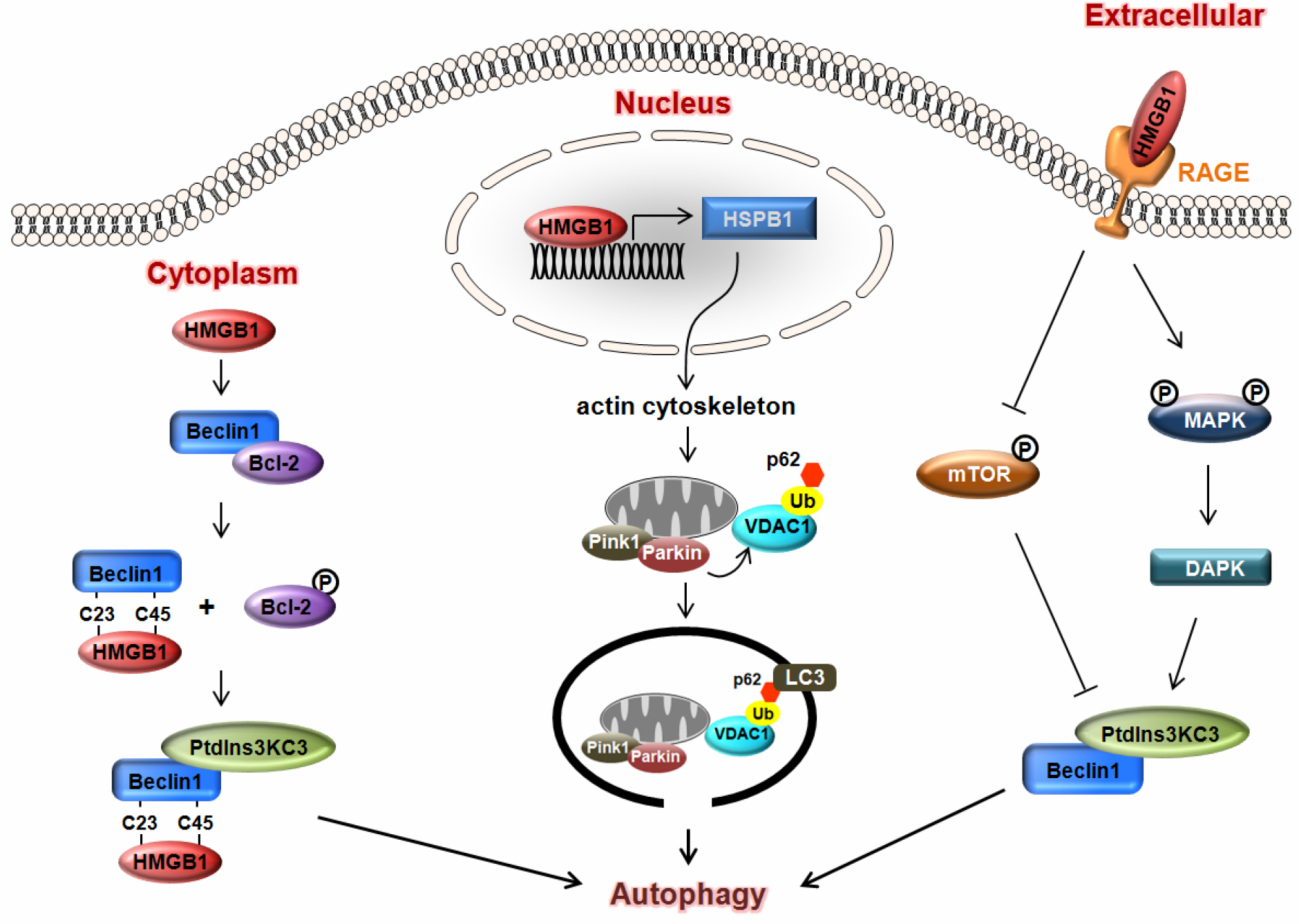
- Reactive oxygen species (ROS) is an oxidative stress to which cells respond by activating various defense mechanisms or cell death. Autophagy associated with oxidative stress response is a process to degrade and recycle macro-molecule as well as organelles in eukaryotic cells. HMGB1, a ubiquitous nuclear protein, is actively released in eukaryotic cells under oxidative stress. HMGB1 plays an important role as regulator of autophagy in nuclear, cytosolic and extracellular level. Nuclear HMGB1 regulates the expression of heat shock protein beta-1 (HSPB1), which is critical for dynamic intracellular trafficking during autophagy and mitophagy. Cytoplasmic HMGB1 can bind to a beclin 1 by the intramolecular disulfide bridge using cysteine 23 and 45, which dissociates its inhibitory partner Bcl-2 and induces autophagy. Extracellular HMGB1 binds to receptor for advanced glycation endproducts (RAGE) which inhibits mammalian target of rapamycin (mTOR) and then promotes the formation of the belin1-Ptdlns3KC3 complex. Furthermore, endogenous HMGB1 is an intrinsic regulator of autophagy, and it enhances chemoresistance in diverse cancer cells. Here, we review recent reports suggesting a novel mechanism of diverse cancer cell resistance to therapy facilitated by HMGB1-mediated autophagy.
Keyword
MeSH Terms
-
Advanced Glycosylation End Product-Specific Receptor
Autophagy
Cell Death
Cysteine
Cytoplasm
Cytosol
Defense Mechanisms
Drug Resistance
Eukaryotic Cells
HMGB1 Protein
HSP27 Heat-Shock Proteins
Mitochondrial Degradation
Nuclear Proteins
Organelles
Oxidative Stress
Reactive Oxygen Species
Receptors, Immunologic
Sirolimus
Cysteine
HMGB1 Protein
HSP27 Heat-Shock Proteins
Nuclear Proteins
Reactive Oxygen Species
Receptors, Immunologic
Sirolimus
Figure
Reference
-
1). Demple B, Amábile-Cuevas CF. Redox redux: the control of oxidative stress responses. Cell. 1991; 67:837–9.
Article2). Grune T, Merker K, Sandig G, Davies KJ. Selective degradation of oxidatively modified protein substrates by the proteasome. Biochem Biophys Res Commun. 2003; 305:709–18.
Article3). Kiffin R, Bandyopadhyay U, Cuervo AM. Oxidative stress and autophagy. Antioxid Redox Signal. 2006; 8:152–62.
Article4). Liang C. Herpesviral interaction with autophagy. J Bacteriol Virol. 2011; 41:213–23.
Article5). Stott K, Watson M, Howe FS, Grossmann JG, Thomas JO. Tail-mediated collapse of HMGB1 is dynamic and occurs via differential binding of the acidic tail to the A and B domains. J Mol Biol. 2010; 403:706–22.
Article6). Hoppe G, Talcott KE, Bhattacharya SK, Crabb JW, Sears JE. Molecular basis for the redox control of nuclear transport of the structural chromatin protein Hmgb1. Exp Cell Res. 2006; 312:3526–38.
Article7). Tang D, Kang R, Zeh HJ 3rd, Lotze MT. High-mobility group box 1 and cancer. Bioichim Biophys Acta. 2010; 1799:131–40.
Article8). Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003; 22:5551–60.
Article9). Youn JH, Shin JS. Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion. J Immunol. 2006; 177:7889–97.
Article10). Oh YJ, Youn JH, Ji Y, Lee SE, Lim KJ, Choi JE, et al. HMGB1 is phosphorylated by classical protein kinase C and is secreted by a calcium-dependent mechanism. J Immunol. 2009; 182:5800–9.
Article11). Ito I, Fukazawa J, Yoshida M. Post-translational methylation of high mobility group Box 1 (HMGB1) causes its cytoplasmic localization in neutrophils. J Biol Chem. 2007; 282:16336–44.
Article12). Tang D, Kang R, Xiao W, Jiang L, Liu M, Shi Y, et al. Nuclear heat shock protein 72 as a negative regulator of oxidative stress (hydrogen peroxide)-induced HMGB1 cytoplasmic translocation and release. J Immunol. 2007; 178:7376–84.
Article13). Lamkanfi M, Sarkar A, Vande Walle L, Vitari AC, Amer AO, Wewers MD, et al. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J Immunol. 2010; 185:4385–92.
Article14). Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002; 418:191–5.
Article15). Bell CW, Jiang W, Reich CF 3rd, Pisetsky DS. The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol. 2006; 291:1318–25.
Article16). Kang R, Livesey KM, Zeh HJ 3rd, Lotze MT, Tang D. Metabolic regulation by HMGB1-mediated autophagy and mitophagy. Autophagy. 2011; 7:1256–8.
Article17). Kang R, Livesey KM, Zeh HJ, Loze MT, Tang D. HMGB1: a novel Beclin 1-binding protein active in autophagy. Autophagy. 2010; 6:1209–11.
Article18). Kang R, Tang D, Loze MT, Zeh HJ. Apoptosis to autophagy switch triggered by the MHC class III-encoded receptor for advanced glycation endproducts (RAGE). Autophagy. 2011; 7:91–3.
Article19). Huang J, Ni J, Liu K, Yu Y, Xie M, Kang R, et al. HMGB1 promotes drug resistance in osteosarcoma. Cancer Res. 2012; 72:230–8.
Article20). Yang L, Yu Y, Kang R, Yang M, Xie M, Wang Z, et al. Up-regulated autophagy by endogenous high mobility group box-1 promotes chemoresistance in leukemia cells. Leuk Lymphoma. 2012; 53:315–22.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Why is autophagy important in human diseases?
- The Role of High Mobility Group Box 1 in Innate Immunity
- Increased Serum High Mobility Group Box 1 (HMGB1) Concentration and the Altered Expression of HMGB1 and Its Receptor Advanced Glycation Endproducts in Pemphigus
- Autophagy in the placenta
- Autophagy and Digestive Disorders: Advances in Understanding and Therapeutic Approaches