J Korean Med Sci.
2014 Nov;29(11):1464-1472. 10.3346/jkms.2014.29.11.1464.
Knockdown of Bcl-xL Enhances Growth-Inhibiting and Apoptosis-Inducing Effects of Resveratrol and Clofarabine in Malignant Mesothelioma H-2452 Cells
- Affiliations
-
- 1Soonchunhyung Environmental Health Center for Asbestos, Soonchunhyang University Cheonan Hospital, Cheonan, Korea. m1037624@sch.ac.kr
- 2Division of Molecular Cancer Research, Soonchunhyang Medical Research Institute, Cheonan, Korea.
- 3Department of Biochemistry, College of Medicine, Soonchunhyang University, Cheonan, Korea.
- 4Department of Urology, Soonchunhyang University Cheonan Hospital, Cheonan, Korea.
- 5Department of Chemistry, Soonchunhyang University, Asan, Korea.
- KMID: 2069926
- DOI: http://doi.org/10.3346/jkms.2014.29.11.1464
Abstract
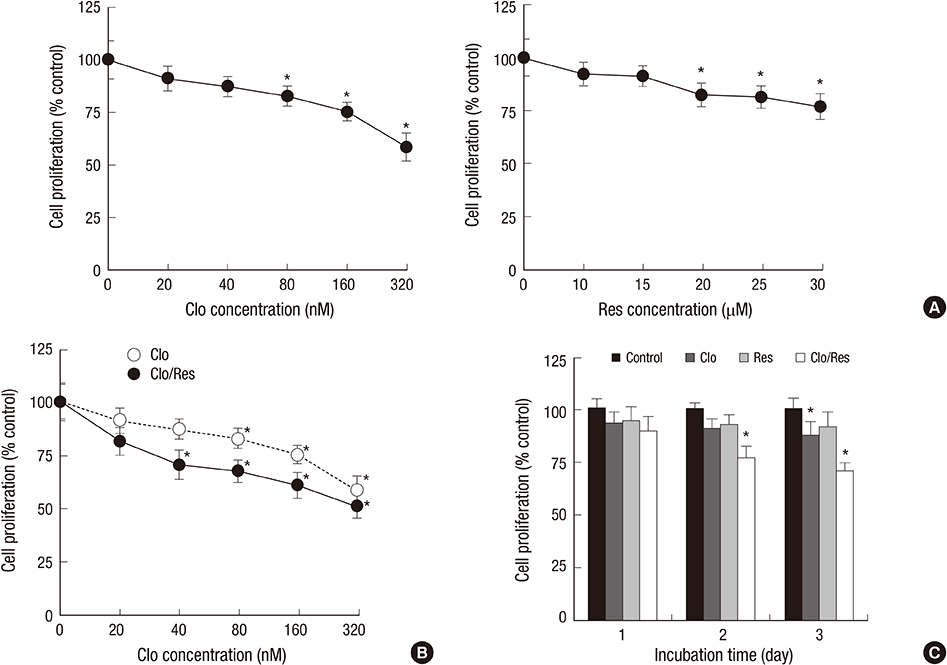
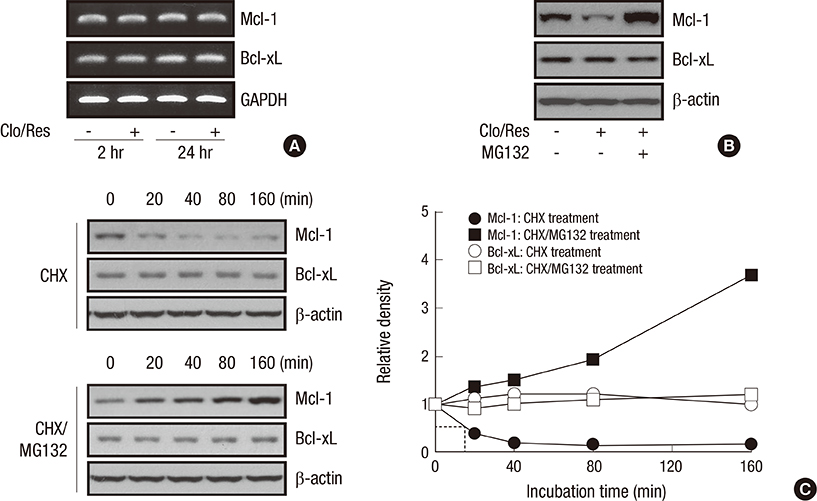
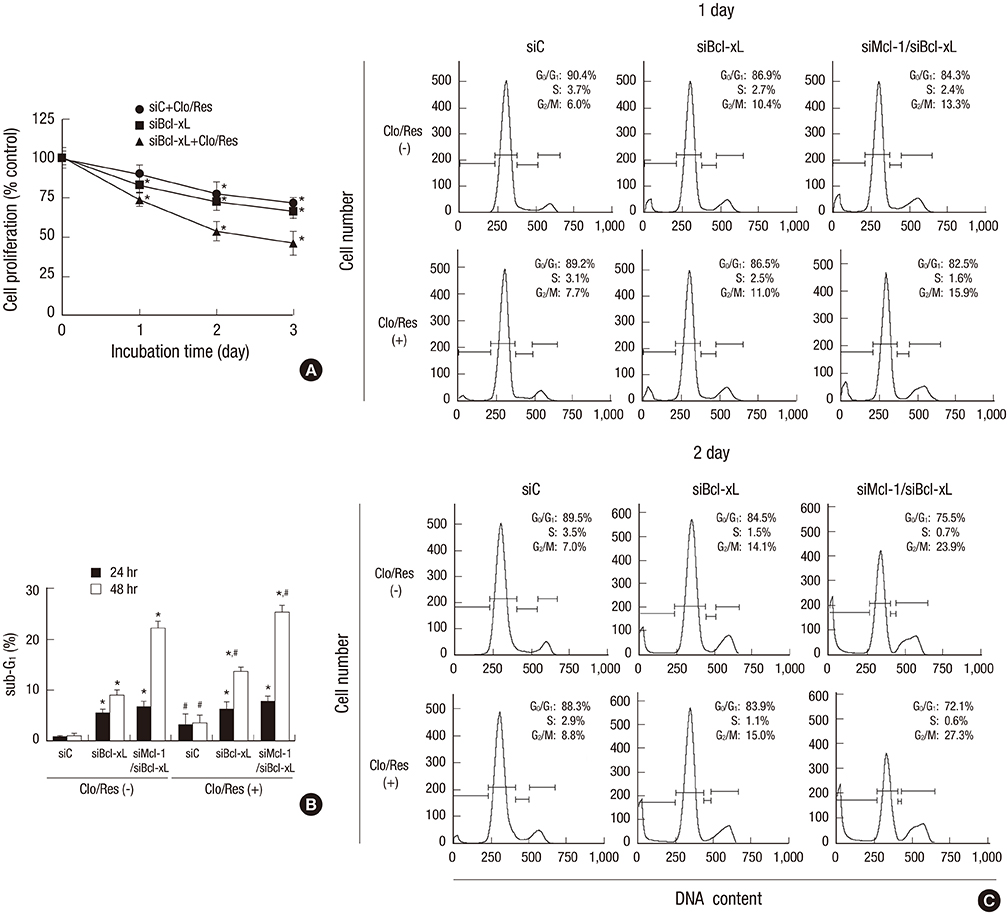
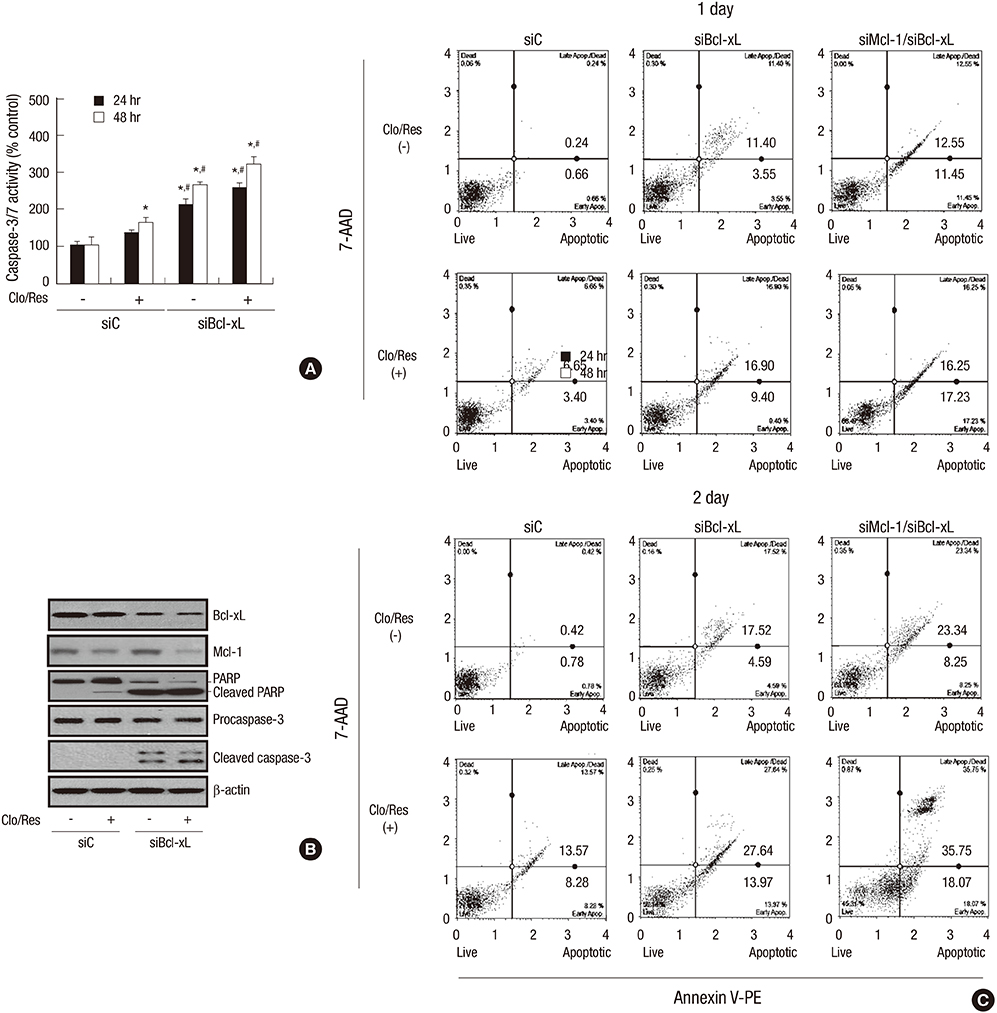
- Mcl-1 and Bcl-xL, key anti-apoptotic proteins of the Bcl-2 family, have attracted attention as important molecules in the cell survival and drug resistance. In this study, we investigated whether inhibition of Bcl-xL influences cell growth and apoptosis against simultaneous treatment of resveratrol and clofarabine in the human malignant mesothelioma H-2452 cells. Resveratrol and clofarabine decreased Mcl-1 protein levels but had little effect on Bcl-xL levels. In the presence of two compounds, any detectable change in the Mcl-1 mRNA levels was not observed in RT-PCR analysis, whereas pretreatment with the proteasome inhibitor MG132 led to its accumulation to levels far above basal levels. The knockdown of Bcl-xL inhibited cell proliferation with cell accumulation at G2/M phase and the appearance of sub-G0/G1 peak in DNA flow cytometric assay. The suppression of cell growth was accompanied by an increase in the caspase-3/7 activity with the resultant cleavages of procaspase-3 and its substrate poly (ADP-ribose) polymerase, and increased percentage of apoptotic propensities in annexin V binding assay. Collectively, our data represent that the efficacy of resveratrol and clofarabine for apoptosis induction was substantially enhanced by Bcl-xL-lowering strategy in which the simultaneous targeting of Mcl-1 and Bcl-xL could be a more effective strategy for treating malignant mesothelioma.
Keyword
MeSH Terms
-
Adenine Nucleotides/*pharmacology
Antimetabolites, Antineoplastic/*pharmacology
Apoptosis/*drug effects
Arabinonucleosides/*pharmacology
Caspase 3/metabolism
Caspase 7/metabolism
Cell Line, Tumor
Cell Proliferation/drug effects
G2 Phase Cell Cycle Checkpoints/drug effects
Gene Knockdown Techniques
Humans
Leupeptins/pharmacology
Lung Neoplasms/metabolism/pathology
M Phase Cell Cycle Checkpoints/drug effects
Mesothelioma/metabolism/pathology
Myeloid Cell Leukemia Sequence 1 Protein/antagonists & inhibitors/genetics/metabolism
RNA Interference
RNA, Messenger/metabolism
RNA, Small Interfering/metabolism
Stilbenes/*pharmacology
bcl-X Protein/antagonists & inhibitors/*genetics/*metabolism
Adenine Nucleotides
Antimetabolites, Antineoplastic
Arabinonucleosides
Leupeptins
Myeloid Cell Leukemia Sequence 1 Protein
RNA, Messenger
RNA, Small Interfering
Stilbenes
bcl-X Protein
Caspase 3
Caspase 7
Figure
Reference
-
1. Grimm D, Wehland M, Pietsch J, Infanger M, Bauer J. Drugs interfering with apoptosis in breast cancer. Curr Pharm Des. 2011; 17:272–283.2. Frenzel A, Grespi F, Chmelewskij W, Villunger A. Bcl2 family proteins in carcinogenesis and the treatment of cancer. Apoptosis. 2009; 14:584–596.3. Kim HR, Heo YM, Jeong KI, Kim YM, Jang HL, Lee KY, Yeo CY, Kim SH, Lee HK, Kim SR, et al. FGF-2 inhibits TNF-alpha mediated apoptosis through upregulation of Bcl2-A1 and Bcl-xL in ATDC5 cells. BMB Rep. 2012; 45:287–292.4. Brotin E, Meryet-Figuière M, Simonin K, Duval RE, Villedieu M, Leroy-Dudal J, Saison-Behmoaras E, Gauduchon P, Denoyelle C, Poulain L. Bcl-XL and MCL-1 constitute pertinent targets in ovarian carcinoma and their concomitant inhibition is sufficient to induce apoptosis. Int J Cancer. 2010; 126:885–895.5. Wuillème-Toumi S, Robillard N, Gomez P, Moreau P, Le Gouill S, Avet-Loiseau H, Harousseau JL, Amiot M, Bataille R. Mcl-1 is overexpressed in multiple myeloma and associated with relapse and shorter survival. Leukemia. 2005; 19:1248–1252.6. Schulze-Bergkamen H, Fleischer B, Schuchmann M, Weber A, Weinmann A, Krammer PH, Galle PR. Suppression of Mcl-1 via RNA interference sensitizes human hepatocellular carcinoma cells towards apoptosis induction. BMC Cancer. 2006; 6:232.7. Karnak D, Xu L. Chemosensitization of prostate cancer by modulating Bcl-2 family proteins. Curr Drug Targets. 2010; 11:699–707.8. Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005; 19:1294–1305.9. Baggstrom MQ, Qi Y, Koczywas M, Argiris A, Johnson EA, Millward MJ, Murphy SC, Erlichman C, Rudin CM, Govindan R. A phase II study of AT-101 (Gossypol) in chemotherapy-sensitive recurrent extensive-stage small cell lung cancer. J Thorac Oncol. 2011; 6:1757–1760.10. Trudel S, Li ZH, Rauw J, Tiedemann RE, Wen XY, Stewart AK. Preclinical studies of the pan-Bcl inhibitor obatoclax (GX015-070) in multiple myeloma. Blood. 2007; 109:5430–5438.11. Soini Y, Kinnula V, Kaarteenaho-Wiik R, Kurttila E, Linnainmaa K, Pääkkö P. Apoptosis and expression of apoptosis regulating proteins bcl-2, mcl-1, bcl-X, and bax in malignant mesothelioma. Clin Cancer Res. 1999; 5:3508–3515.12. Montanaro F, Rosato R, Gangemi M, Roberti S, Ricceri F, Merler E, Gennaro V, Romanelli A, Chellini E, Pascucci C, et al. Survival of pleural malignant mesothelioma in Italy: a population-based study. Int J Cancer. 2009; 124:201–207.13. Wilson TR, Johnston PG, Longley DB. Anti-apoptotic mechanisms of drug resistance in cancer. Curr Cancer Drug Targets. 2009; 9:307–319.14. Lee YJ, Park IS, Lee YJ, Shim JH, Cho MK, Nam HS, Park JW, Oh MH, Lee SH. Resveratrol contributes to chemosensitivity of malignant mesothelioma cells with activation of p53. Food Chem Toxicol. 2014; 63:153–160.15. Lee YJ, Im JH, Lee DM, Park JS, Won SY, Cho MK, Nam HS, Lee YJ, Lee SH. Synergistic inhibition of mesothelioma cell growth by the combination of clofarabine and resveratrol involves Nrf2 downregulation. BMB Rep. 2012; 45:647–652.16. Lee YJ, Lee YJ, Im JH, Won SY, Kim YB, Cho MK, Nam HS, Choi YJ, Lee SH. Synergistic anti-cancer effects of resveratrol and chemotherapeutic agent clofarabine against human malignant mesothelioma MSTO-211H cells. Food Chem Toxicol. 2013; 52:61–68.17. Craig RW. MCL1 provides a window on the role of the BCL2 family in cell proliferation, differentiation and tumorigenesis. Leukemia. 2002; 16:444–454.18. Derouet M, Thomas L, Cross A, Moots RJ, Edwards SW. Granulocyte macrophage colony-stimulating factor signaling and proteasome inhibition delay neutrophil apoptosis by increasing the stability of Mcl-1. J Biol Chem. 2004; 279:26915–26921.19. Nencioni A, Hua F, Dillon CP, Yokoo R, Scheiermann C, Cardone MH, Barbieri E, Rocco I, Garuti A, Wesselborg S, et al. Evidence for a protective role of Mcl-1 in proteasome inhibitor-induced apoptosis. Blood. 2005; 105:3255–3262.20. Nijhawan D, Fang M, Traer E, Zhong Q, Gao W, Du F, Wang X. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 2003; 17:1475–1486.21. Song L, Coppola D, Livingston S, Cress D, Haura EB. Mcl-1 regulates survival and sensitivity to diverse apoptotic stimuli in human non-small cell lung cancer cells. Cancer Biol Ther. 2005; 4:267–276.22. Pohl J, Zuna I, Stremmel W, Rudi J. Systemic chemotherapy with epirubicin for treatment of advanced or multifocal hepatocellular carcinoma. Chemotherapy. 2001; 47:359–365.23. Shimazu T, Degenhardt K, Nur-E-Kamal A, Zhang J, Yoshida T, Zhang Y, Mathew R, White E, Inouye M. NBK/BIK antagonizes MCL-1 and BCL-XL and activates BAK-mediated apoptosis in response to protein synthesis inhibition. Genes Dev. 2007; 21:929–941.24. Takahashi H, Chen MC, Pham H, Matsuo Y, Ishiguro H, Reber HA, Takeyama H, Hines OJ, Eibl G. Simultaneous knock-down of Bcl-xL and Mcl-1 induces apoptosis through Bax activation in pancreatic cancer cells. Biochim Biophys Acta. 2013; 1833:2980–2987.25. Pal HC, Sharma S, Elmets CA, Athar M, Afaq F. Fisetin inhibits growth, induces G(2)/M arrest and apoptosis of human epidermoid carcinoma A431 cells: role of mitochondrial membrane potential disruption and consequent caspases activation. Exp Dermatol. 2013; 22:470–475.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Preventive Effects of Bcl-2 and Bcl-xL on Lovastatin-induced Apoptosis of C6 Glial Cells
- Apoptosis of Prostate Cancer by Inhibition of Bcl-xL Expression
- Antiproliferative and Cytotoxic Effects of Resveratrol in Mitochondria-Mediated Apoptosis in Rat B103 Neuroblastoma Cells
- Running Title: Apoptotic Effect of Mycolactone in SCC15 Cells
- Fluoxetine Up-Regulates Bcl-xL Expression in Rat C6 Glioma Cells