Chonnam Med J.
2011 Aug;47(2):104-110. 10.4068/cmj.2011.47.2.104.
Theaflavin Inhibits LPS-Induced IL-6, MCP-1, and ICAM-1 Expression in Bone Marrow-Derived Macrophages Through the Blockade of NF-kappaB and MAPK Signaling Pathways
- Affiliations
-
- 1Korean Minjok Leadership Academy, Hoengseong, Korea.
- 2Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea. yejoo@chonnam.ac.kr
- KMID: 2048793
- DOI: http://doi.org/10.4068/cmj.2011.47.2.104
Abstract
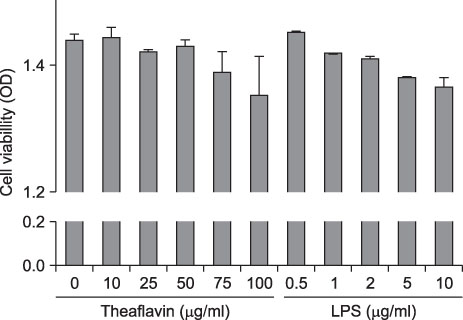
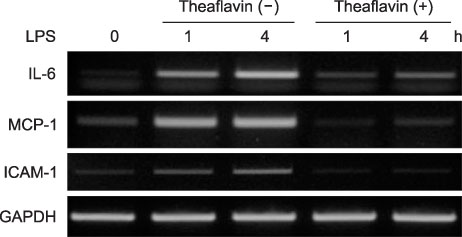
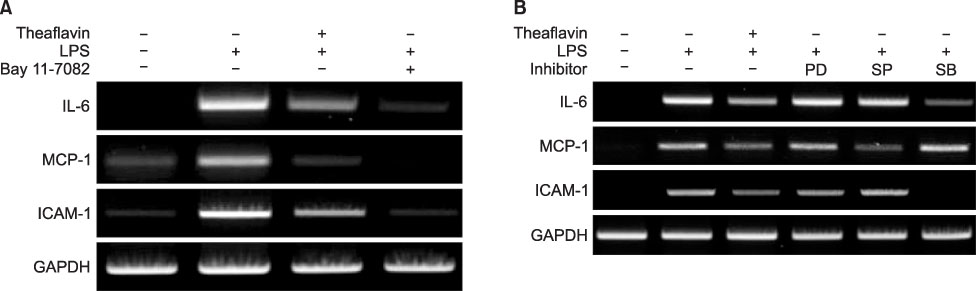
- Theaflavin, the main polyphenol in black tea, has anti-inflammatory, antioxidative, anti-mutagenic, and anti-carcinogenic properties. The aim of this study was to evaluate the effects of theaflavin on lipopolysaccharide (LPS)-induced innate signaling and expression of pro-inflammatory mediators in bone marrow-derived macrophages isolated from ICR mice. The effects of theaflavin on the expression of proinflammatory mediators, LPS-induced nuclear factor-kappa B (NF-kappaB), and mitogen-activated protein kinase (MAPK) signaling pathways were examined by reverse transcriptase-polymerase chain reaction (RT-PCR), Western blotting, and immunofluorescence. LPS-induced interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), and intercellular adhesion molecule-1 (ICAM-1) expression was inhibited by theaflavin. LPS-induced inhibitor kappa B alpha (IkappaBalpha) degradation and nuclear translocation of RelA were blocked by theaflavin. LPS-induced phosphorylation of extracellular signal-regulated kinase1/2 (ERK1/2), c-Jun-N-terminal kinase (JNK), and p38 MAPK was inhibited by theaflavin. The inhibitory effect of theaflavin on IL-6, MCP-1, and ICAM-1 expression was completely inhibited by Bay11-7082 (NF-kappaB inhibitor). The inhibitory effect of theaflavin on IL-6 and ICAM-1 expression was inhibited by SB203580 (p38 MAPK inhibitor). The inhibitory effect of theaflavin on MCP-1 expression was inhibited by SP600125 (JNK inhibitor). These results indicate that theaflavin prevents LPS-induced IL-6, MCP-1, and ICAM-1 expression through blockade of NF-kappaB and MAPK signaling pathways in bone marrow-derived macrophages.
Keyword
MeSH Terms
-
Animals
Anthracenes
Biflavonoids
Blotting, Western
Catechin
Chemokine CCL2
Fluorescent Antibody Technique
Imidazoles
Intercellular Adhesion Molecule-1
Interleukin-6
Macrophages
Mice
Mice, Inbred ICR
NF-kappa B
Nitriles
p38 Mitogen-Activated Protein Kinases
Phosphorylation
Phosphotransferases
Protein Kinases
Pyridines
Sulfones
Tea
Anthracenes
Biflavonoids
Catechin
Chemokine CCL2
Imidazoles
Intercellular Adhesion Molecule-1
Interleukin-6
NF-kappa B
Nitriles
Phosphotransferases
Protein Kinases
Pyridines
Sulfones
Tea
p38 Mitogen-Activated Protein Kinases
Figure
Reference
-
1. Khan N, Mukhtar H. Tea polyphenols for health promotion. Life Sci. 2007. 81:519–533.
Article2. Khan N, Mukhtar H. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett. 2008. 269:269–280.
Article3. Sharma V, Rao LJ. A thought on the biological activities of black tea. Crit Rev Food Sci Nutr. 2009. 49:379–404.
Article4. Aneja R, Odoms K, Denenberg AG, Wong HR. Theaflavin, a black tea extract, is a novel anti-inflammatory compound. Crit Care Med. 2004. 32:2097–2103.
Article5. Ukil A, Maity S, Das PK. Protection from experimental colitis by theaflavin-3,3'-digallate correlates with inhibition of IKK and NF-kappaB activation. Br J Pharmacol. 2006. 149:121–131.
Article6. Gosslau A, En Jao DL, Huang MT, Ho CT, Evans D, Rawson NE, et al. Effects of the black tea polyphenol theaflavin-2 on apoptotic and inflammatory pathways in vitro and in vivo. Mol Nutr Food Res. 2011. 55:198–208.
Article7. Cai F, Li C, Wu J, Min Q, Ouyang C, Zheng M, et al. Modulation of the oxidative stress and nuclear factor kappaB activation by theaflavin 3,3'-gallate in the rats exposed to cerebral ischemia-reperfusion. Folia Biol (Praha). 2007. 53:164–172.8. Huang MT, Liu Y, Ramji D, Lo CY, Ghai G, Dushenkov S, et al. Inhibitory effects of black tea theaflavin derivatives on 12-O-tetradecanoylphorbol-13-acetate-induced inflammation and arachidonic acid metabolism in mouse ears. Mol Nutr Food Res. 2006. 50:115–122.
Article9. Maity S, Ukil A, Karmakar S, Datta N, Chaudhuri T, Vedasiromoni JR, et al. Thearubigin, the major polyphenol of black tea, ameliorates mucosal injury in trinitrobenzene sulfonic acid-induced colitis. Eur J Pharmacol. 2003. 470:103–112.
Article10. Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007. 117:514–521.
Article11. Rose-John S, Mitsuyama K, Matsumoto S, Thaiss WM, Scheller J. Interleukin-6 trans-signaling and colonic cancer associated with inflammatory bowel disease. Curr Pharm Des. 2009. 15:2095–2103.
Article12. Scheller J, Ohnesorge N, Rose-John S. Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand J Immunol. 2006. 63:321–329.
Article13. Yadav A, Saini V, Arora S. MCP-1: chemoattractant with a role beyond immunity: a review. Clin Chim Acta. 2010. 411:1570–1579.
Article14. Vainer B. Intercellular adhesion molecule-1 (ICAM-1) in ulcerative colitis: presence, visualization, and significance. APMIS Suppl. 2010. 129:1–43.
Article15. Hall DJ, Bates ME, Guar L, Cronan M, Korpi N, Bertics PJ. The role of p38 MAPK in rhinovirus-induced monocyte chemoattractant protein-1 production by monocytic-lineage cells. J Immunol. 2005. 174:8056–8063.
Article16. Perkins ND, Gilmore TD. Good cop, bad cop: the different faces of NF-kappaB. Cell Death Differ. 2006. 13:759–772.17. Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004. 18:2195–2224.18. Kong AN, Yu R, Chen C, Mandlekar S, Primiano T. Signal transduction events elicited by natural products: role of MAPK and caspase pathways in homeostatic response and induction of apoptosis. Arch Pharm Res. 2000. 23:1–16.
Article19. Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995. 9:726–735.
Article20. Chambers TJ, Owens JM, Hattersley G, Jat PS, Noble MD. Generation of osteoclast-inductive and osteoclastogenic cell lines from the H-2KbtsA58 transgenic mouse. Proc Natl Acad Sci U S A. 1993. 90:5578–5582.
Article21. Aneja R, Odoms K, Denenberg AG, Wong HR. Theaflavin, a black tea extract, is a novel anti-inflammatory compound. Crit Care Med. 2004. 32:2097–2103.
Article22. Pan MH, Lin-Shiau SY, Ho CT, Lin JH, Lin JK. Suppression of lipopolysaccharide-induced nuclear factor-kappaB activity by theaflavin-3,3'-digallate from black tea and other polyphenols through down-regulation of IkappaB kinase activity in macrophages. Biochem Pharmacol. 2000. 59:357–367.
Article23. Nomura M, Ma W, Chen N, Bode AM, Dong Z. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced NF-kappaB activation by tea polyphenols, (-)-epigallocatechin gallate and theaflavins. Carcinogenesis. 2000. 21:1885–1890.
Article24. Kong AN, Owuor E, Yu R, Hebbar V, Chen C, Hu R, et al. Induction of xenobiotic enzymes by the MAP kinase pathway and the antioxidant or electrophile response element (ARE/EpRE). Drug Metab Rev. 2001. 33:255–271.
Article25. Van Den Blink B, Ten Hove T, Van Den Brink GR, Peppelenbosch MP, Van Deventer SJ. From extracellular to intracellular targets, inhibiting MAP kinases in treatment of Crohn's disease. Ann N Y Acad Sci. 2002. 973:349–358.
Article26. Broom OJ, Widjaya B, Troelsen J, Olsen J, Nielsen OH. Mitogen activated protein kinases: a role in inflammatory bowel disease? Clin Exp Immunol. 2009. 158:272–280.
Article27. Kalra N, Seth K, Prasad S, Singh M, Pant AB, Shukla Y. Theaflavins induced apoptosis of LNCaP cells is mediated through induction of p53, down-regulation of NF-kappa B and mitogen-activated protein kinases pathways. Life Sci. 2007. 80:2137–2146.
Article28. Bhattacharya U, Halder B, Mukhopadhyay S, Giri AK. Role of oxidation-triggered activation of JNK and p38 MAPK in black tea polyphenols induced apoptotic death of A375 cells. Cancer Sci. 2009. 100:1971–1978.
Article29. Siddiqui IA, Adhami VM, Afaq F, Ahmad N, Mukhtar H. Modulation of phosphatidylinositol-3-kinase/protein kinase B- and mitogen-activated protein kinase-pathways by tea polyphenols in human prostate cancer cells. J Cell Biochem. 2004. 91:232–242.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epigallocatechin-3-gallate Inhibits LPS-Induced NF-kappaB and MAPK Signaling Pathways in Bone Marrow-Derived Macrophages
- Blockade of p38 Mitogen-activated Protein Kinase Pathway Inhibits Interleukin-6 Release and Expression in Primary Neonatal Cardiomyocytes
- Mycobacterial Heparin-binding Hemagglutinin Antigen Activates Inflammatory Responses through PI3-K/Akt, NF-kappaB, and MAPK Pathways
- Silymarin Inhibits Morphological Changes in LPS-Stimulated Macrophages by Blocking NF-kappaB Pathway
- Epigallocatechin-3-gallate Inhibits the Expression of Adhesion Molecules by Blocking Nuclear Factor Kappa B Signaling in Intestinal Epithelial Cells