J Korean Med Sci.
2016 Sep;31(9):1419-1425. 10.3346/jkms.2016.31.9.1419.
Metformin Reduces Bleomycin-induced Pulmonary Fibrosis in Mice
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea. ywkim@snu.ac.kr
- 2Department of Pathology, Seoul National University Hospital, Seoul, Korea.
- KMID: 2468274
- DOI: http://doi.org/10.3346/jkms.2016.31.9.1419
Abstract
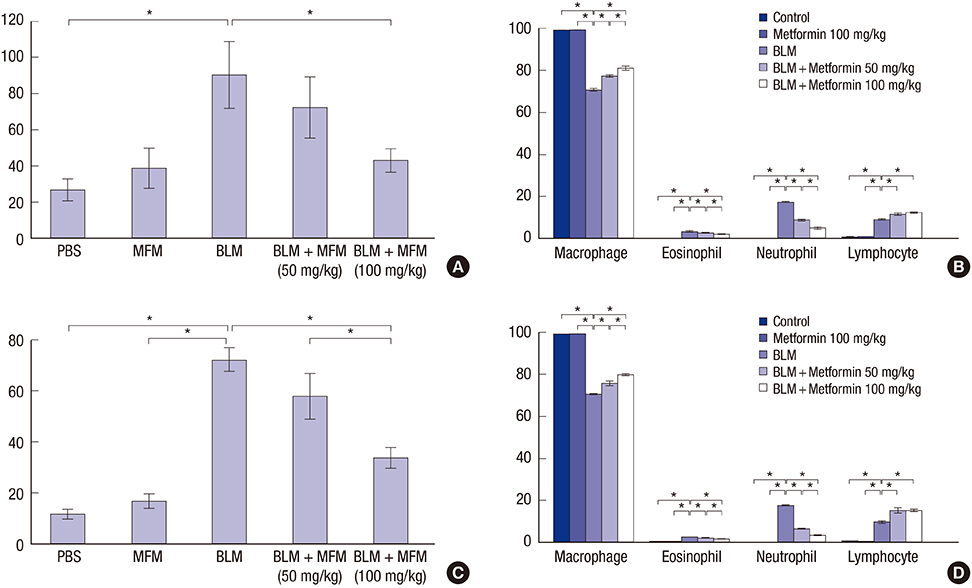
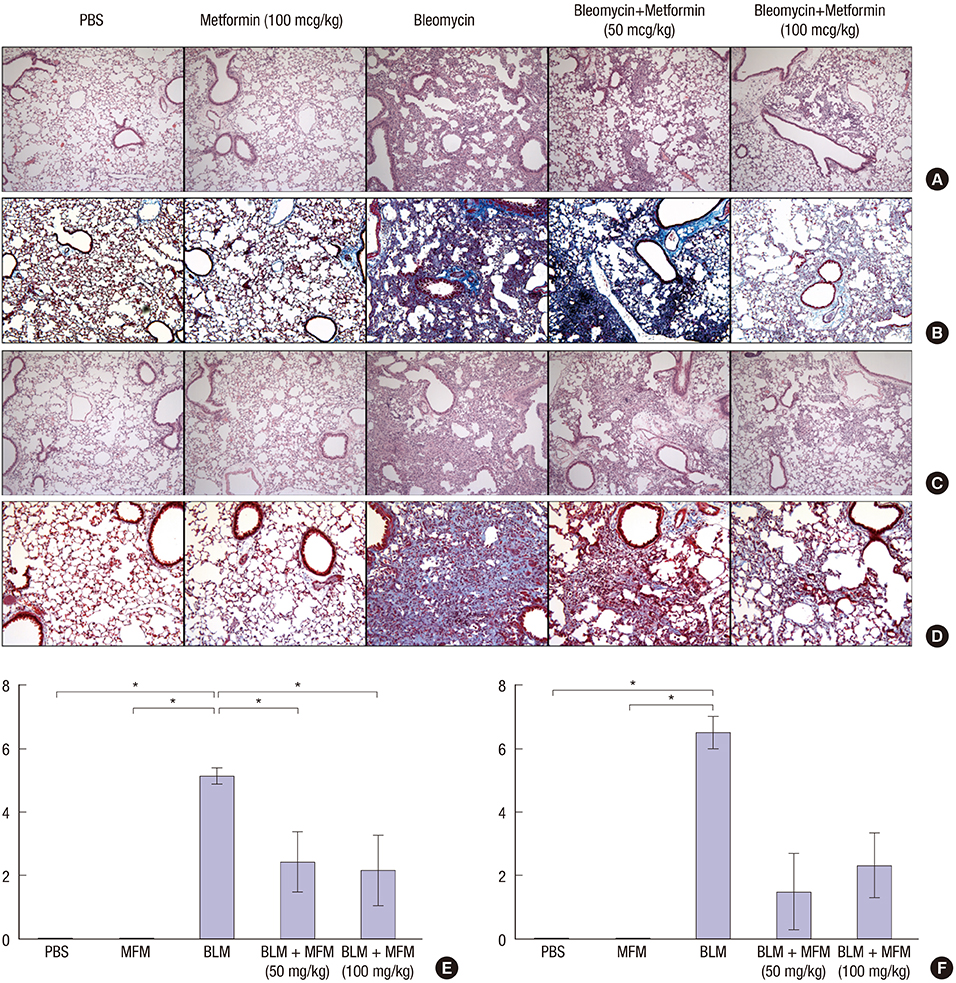
- Metformin has anti-inflammatory and anti-fibrotic effects. We investigated whether metformin has an inhibitory effect on bleomycin (BLM)-induced pulmonary fibrosis in a murine model. A total of 62 mice were divided into 5 groups: control, metformin (100 mg/kg), BLM, and BLM with metformin (50 mg/kg or 100 mg/kg). Metformin was administered to the mice orally once a day from day 1. We sacrificed half of the mice on day 10 and collected the bronchoalveolar lavage fluid (BALF) from their left lungs. The remaining mice were sacrificed and analyzed on day 21. The right lungs were harvested for histological analyses. The messenger RNA (mRNA) levels of epithelial-mesenchymal transition markers were determined via analysis of the harvested lungs on day 21. The mice treated with BLM and metformin (50 mg/kg or 100 mg/kg) showed significantly lower levels of inflammatory cells in the BALF compared with the BLM-only mice on days 10 and 21. The histological examination revealed that the metformin treatment led to a greater reduction in inflammation than the treatment with BLM alone. The mRNA levels of collagen, collagen-1, procollagen, fibronectin, and transforming growth factor-β in the metformin-treated mice were lower than those in the BLM-only mice on day 21, although statistical significance was observed only in the case of procollagen due to the small number of live mice in the BLM-only group. Additionally, treatment with metformin reduced fibrosis to a greater extent than treatment with BLM alone. Metformin suppresses the inflammatory and fibrotic processes of BLM-induced pulmonary fibrosis in a murine model.
Keyword
MeSH Terms
-
Animals
Bleomycin/toxicity
Bronchoalveolar Lavage Fluid/chemistry
Collagen Type I/genetics/metabolism
Dose-Response Relationship, Drug
Female
Fibronectins/genetics/metabolism
Lung/pathology
Metformin/*therapeutic use
Mice
Mice, Inbred C57BL
Pulmonary Fibrosis/chemically induced/*prevention & control
RNA, Messenger/metabolism
Tumor Necrosis Factor-alpha/metabolism
Collagen Type I
Fibronectins
RNA, Messenger
Tumor Necrosis Factor-alpha
Bleomycin
Metformin
Figure
Cited by 1 articles
-
Role of nuclear factor-kappa B in bleomycin induced pulmonary fibrosis and the probable alleviating role of ginsenoside: histological, immunohistochemical, and biochemical study
Dalia Refaat El-Bassouny, Nesreen Mostafa Omar, Hanaa Attia Khalaf, Reem Ahmad Abd Al-Salam
Anat Cell Biol. 2021;54(4):448-464. doi: 10.5115/acb.21.068.
Reference
-
1. Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007; 117:524–529.2. Lee CM, Park JW, Cho WK, Zhou Y, Han B, Yoon PO, Chae J, Elias JA, Lee CG. Modifiers of TGF-beta1 effector function as novel therapeutic targets of pulmonary fibrosis. Korean J Intern Med. 2014; 29:281–290.3. Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol. 2008; 40:362–382.4. Park CS, Bang BR, Kwon HS, Moon KA, Kim TB, Lee KY, Moon HB, Cho YS. Metformin reduces airway inflammation and remodeling via activation of AMP-activated protein kinase. Biochem Pharmacol. 2012; 84:1660–1670.5. Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988; 41:467–470.6. Xiao H, Ma X, Feng W, Fu Y, Lu Z, Xu M, Shen Q, Zhu Y, Zhang Y. Metformin attenuates cardiac fibrosis by inhibiting the TGFbeta1-Smad3 signalling pathway. Cardiovasc Res. 2010; 87:504–513.7. Bai J, Zhang N, Hua Y, Wang B, Ling L, Ferro A, Xu B. Metformin inhibits angiotensin II-induced differentiation of cardiac fibroblasts into myofibroblasts. PLoS One. 2013; 8:e72120.8. Vasquez C, Benamer N, Morley GE. The cardiac fibroblast: functional and electrophysiological considerations in healthy and diseased hearts. J Cardiovasc Pharmacol. 2011; 57:380–388.9. Kim H, Moon SY, Kim JS, Baek CH, Kim M, Min JY, Lee SK. Activation of AMP-activated protein kinase inhibits ER stress and renal fibrosis. Am J Physiol Renal Physiol. 2015; 308:F226–36.10. Zhang X, Harmsen WS, Mettler TA, Kim WR, Roberts RO, Therneau TM, Roberts LR, Chaiteerakij R. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology. 2014; 60:2008–2016.11. Kita Y, Takamura T, Misu H, Ota T, Kurita S, Takeshita Y, Uno M, Matsuzawa-Nagata N, Kato K, Ando H, et al. Metformin prevents and reverses inflammation in a non-diabetic mouse model of nonalcoholic steatohepatitis. PLoS One. 2012; 7:e43056.12. Garinis GA, Fruci B, Mazza A, De Siena M, Abenavoli S, Gulletta E, Ventura V, Greco M, Abenavoli L, Belfiore A. Metformin versus dietary treatment in nonalcoholic hepatic steatosis: a randomized study. Int J Obes. 2010; 34:1255–1264.13. Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, David E, Rizzetto M, Marchesini G. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005; 100:1082–1090.14. Chaudhary NI, Schnapp A, Park JE. Pharmacologic differentiation of inflammation and fibrosis in the rat bleomycin model. Am J Respir Crit Care Med. 2006; 173:769–776.15. Mouratis MA, Aidinis V. Modeling pulmonary fibrosis with bleomycin. Curr Opin Pulm Med. 2011; 17:355–361.16. Song P, Zou MH. Regulation of NAD(P)H oxidases by AMPK in cardiovascular systems. Free Radic Biol Med. 2012; 52:1607–1619.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Deficiency of Sphingosine-1-Phosphate Receptor 2 (S1Pâ‚‚) Attenuates Bleomycin-Induced Pulmonary Fibrosis
- Experimental Study of the Pulmonary Toxicity of Combined Bleomycin and Captopril Administration in Mice
- Utility of Micro CT in a Murine Model of Bleomycin-Induced Lung Fibrosis
- Hemin attenuates bleomycin-induced lung fibrosis in mice by regulating the TGF-ββ1/MAPK and AMPK/SIRT1/PGC-1αα/HO-1/ NF-κκB pathways
- A review of current studies on cellular and molecular mechanisms underlying pulmonary fibrosis induced by chemicals