Anat Cell Biol.
2019 Sep;52(3):302-311. 10.5115/acb.18.180.
Improvement in histology, enzymatic activity, and redox state of the liver following administration of Cinnamomum zeylanicum bark oil in rats with established hepatotoxicity
- Affiliations
-
- 1Cellular and Molecular Research Center, Faculty of Medicine, Guilan University of Medical Sciences, Rasht, Iran. ebrahimnasiri@gmail.com
- 2Department of Anatomical Sciences, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
- 3Student Research Committee, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
- 4Department of Pharmacognosy, School of Pharmacy, Guilan University of Medical Sciences, Rasht, Iran.
- KMID: 2459546
- DOI: http://doi.org/10.5115/acb.18.180
Abstract
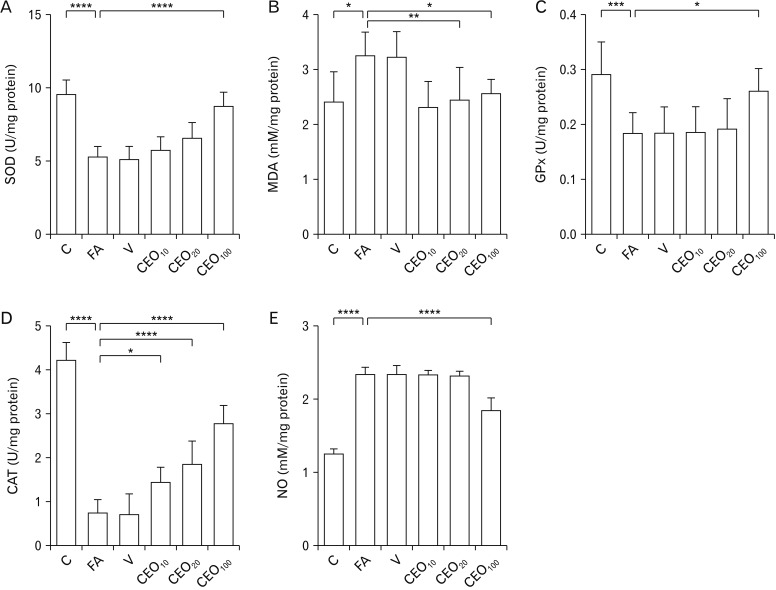
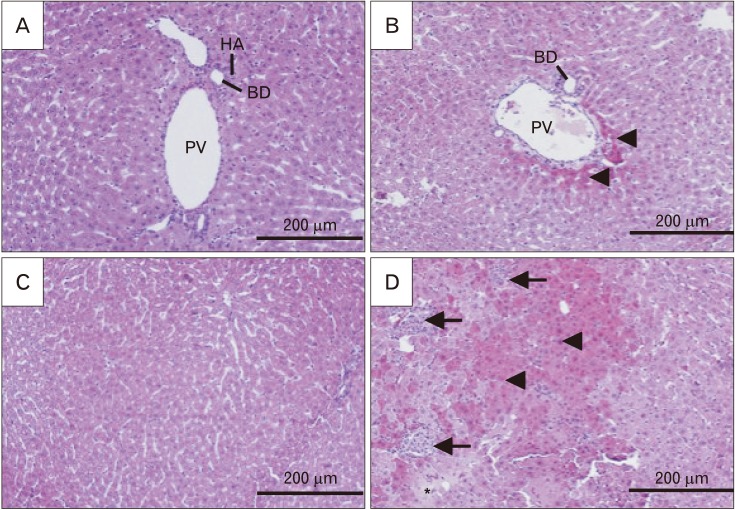
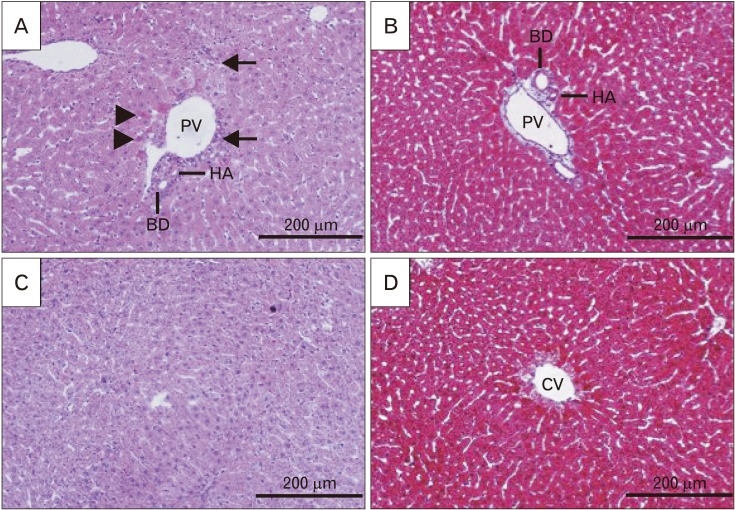
- Formaldehyde (FA) is an environmentally-available pollutant. Since the liver acts as a detoxifier in the human body, it is the first and most affected organ in individuals exposed to higher-than-normal amounts of FA. FA mainly alters oxidant/antioxidant status and initiates oxidative stress, and by means, causes functional damage to the liver. Thus, it is important to identify natural bioactive compounds with antioxidant properties in order to be used as food additives. Cinnamon (Cinnamomum zeylanicum) is a popular flavor and also a medicinal plant with a variety of beneficial effects. In the present original study, cinnamon essential oil (CEO) has been administrated at doses of 10, 20, and 100 mg/kg, orally, to hepatotoxicity rat models caused by FA (10 mg/kg, intraperitoneally). Liver enzymes and its histology were assessed and oxidative stress biomarkers in the liver tissue were also examined. CEO administration caused a significant increase in superoxide dismutase, glutathione peroxidase, and catalase and a prominent decrease in nitric oxide levels in the liver tissue. Also, in serum samples, CEO significantly reduced the elevated amounts of alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase. When assessed histologically, portal area and central vein fibrosis alongside with the hepatocytes' hypereosinophilia and swelling, focal inflammation, and necrotic areas were found to be prominently decreased in the CEO group. In conclusion, our study suggested that the CEO may have the potential for being used against FA-induced hepatotoxicity.
MeSH Terms
-
Alanine Transaminase
Alkaline Phosphatase
Animals
Antioxidants
Aspartate Aminotransferases
Biomarkers
Catalase
Cinnamomum zeylanicum*
Cinnamomum*
Fibrosis
Food Additives
Formaldehyde
Glutathione Peroxidase
Human Body
Inflammation
Liver*
Models, Animal
Nitric Oxide
Oxidation-Reduction*
Oxidative Stress
Plants, Medicinal
Rats*
Superoxide Dismutase
Veins
Alanine Transaminase
Alkaline Phosphatase
Antioxidants
Aspartate Aminotransferases
Biomarkers
Catalase
Food Additives
Formaldehyde
Glutathione Peroxidase
Nitric Oxide
Superoxide Dismutase
Figure
Cited by 1 articles
-
Inhibitory effect of temozolomide on apoptosis induction of cinnamaldehyde in human glioblastoma multiforme T98G cell line
Hedieh Abband, Sara Dabirian, Adele Jafari, Mehran Nasiri, Ebrahim Nasiri
Anat Cell Biol. 2024;57(1):85-96. doi: 10.5115/acb.23.159.
Reference
-
1. Molina DK, DiMaio VJ. Normal organ weights in men: part II-the brain, lungs, liver, spleen, and kidneys. Am J Forensic Med Pathol. 2012; 33:368–372. PMID: 22182984.2. Molina DK, DiMaio VJ. Normal organ weights in women: part II. the brain, lungs, liver, spleen, and kidneys. Am J Forensic Med Pathol. 2015; 36:182–187. PMID: 26108038.3. Gil MN, Choi DR, Yu KS, Jeong JH, Bak DH, Kim DK, Lee NS, Lee JH, Jeong YG, Na CS, Na DS, Ryu KH, Han SY. Rhus verniciflua stokes attenuates cholestatic liver cirrhosis-induced interstitial fibrosis via Smad3 down-regulation and Smad7 up-regulation. Anat Cell Biol. 2016; 49:189–198. PMID: 27722012.4. Bhattacharya A, Dhar P, Mehra RD. Preliminary morphological and biochemical changes in rat liver following postnatal exposure to sodium arsenite. Anat Cell Biol. 2012; 45:229–240. PMID: 23301191.5. Saowakon N, Ngernsoungnern P, Watcharavitoon P, Ngernsoungnern A, Kosanlavit R. Formaldehyde exposure in gross anatomy laboratory of Suranaree University of Technology: a comparison of area and personal sampling. Environ Sci Pollut Res Int. 2015; 22:19002–19012. PMID: 26233735.6. Bunyavaree M, Kasemsarn P, Boonchai W. Cosmetic preservative labelling on the Thai market. Contact Dermatitis. 2016; 74:217–221. PMID: 26799537.7. Ou LT. Enhanced degradation of the volatile fumigant-nematicides 1,3-d and methyl bromide in soil. J Nematol. 1998; 30:56–64. PMID: 19274199.8. O'Quinn SE, Kennedy CB. Contact dermatitis due to formaldehyde in clothing textiles. JAMA. 1965; 194:593–596. PMID: 5897231.9. Viegas S, Ladeira C, Nunes C, Malta-Vacas J, Gomes M, Brito M, Mendonca P, Prista J. Genotoxic effects in occupational exposure to formaldehyde: a study in anatomy and pathology laboratories and formaldehyde-resins production. J Occup Med Toxicol. 2010; 5:25. PMID: 20727169.10. Kotzias D, Geiss O, Tirendi S, Barrero-Moreno J, Reina V, Gotti A, Cimino-Reale G, Casati B, Marafante E, Sarigiannis D. Exposure to multiple air contaminants in public buildings, schools and kindergartens: The European Indoor Air Monitoring and Exposure Assessment Study (AIRMEX) Study. Fresen Environ Bull. 2009; 18:670–681.11. Teng S, Beard K, Pourahmad J, Moridani M, Easson E, Poon R, O'Brien PJ. The formaldehyde metabolic detoxification enzyme systems and molecular cytotoxic mechanism in isolated rat hepatocytes. Chem Biol Interact. 2001; 130-132:285–296. PMID: 11306052.12. Beall JR, Ulsamer AG. Formaldehyde and hepatotoxicity: a review. J Toxicol Environ Health. 1984; 14:1–21. PMID: 6389892.13. Matsuoka T, Takaki A, Ohtaki H, Shioda S. Early changes to oxidative stress levels following exposure to formaldehyde in ICR mice. J Toxicol Sci. 2010; 35:721–730. PMID: 20930466.14. Talaei B, Amouzegar A, Sahranavard S, Hedayati M, Mirmiran P, Azizi F. Effects of cinnamon consumption on glycemic indicators, advanced glycation end products, and antioxidant status in type 2 diabetic patients. Nutrients. 2017; 9:E991. PMID: 28885566.15. Noudeh GD, Sharififar F, Noodeh AD, Moshafi MH, Afzadi MA, Behravan E, Aref M, Sakhtianchi R. Antitumor and antibacterial activity of four fractions from Heracleum persicum Desf. and Cinnamomum zeylanicum Blume. J Med Plants Res. 2010; 4:2176–2180.16. Sambaiah K, Srinivasan K. Effect of cumin, cinnamon, ginger, mustard and tamarind in induced hypercholesterolemic rats. Nahrung. 1991; 35:47–51. PMID: 1865890.17. Eidi A, Mortazavi P, Bazargan M, Zaringhalam J. Hepatoprotective activity of cinnamon ethanolic extract against CCI4-induced liver injury in rats. EXCLI J. 2012; 11:495–507. PMID: 27547174.18. Gunawardena D, Karunaweera N, Lee S, van Der Kooy F, Harman DG, Raju R, Bennett L, Gyengesi E, Sucher NJ, Münch G. Anti-inflammatory activity of cinnamon (C. zeylanicum and C. cassia) extracts: identification of E-cinnamaldehyde and o-methoxy cinnamaldehyde as the most potent bioactive compounds. Food Funct. 2015; 6:910–919. PMID: 25629927.19. Maisonneune SA. European Pharmacopoeia, Vol. 3. Saint-Ruffine: Maisonneuve;1975.20. Malik J, Munjal K, Deshmukh R. Attenuating effect of standardized lyophilized Cinnamomum zeylanicum bark extract against streptozotocin-induced experimental dementia of Alzheimer's type. J Basic Clin Physiol Pharmacol. 2015; 26:275–285. PMID: 25301673.21. Jain S, Sangma T, Shukla SK, Mediratta PK. Effect of Cinnamomum zeylanicum extract on scopolamine-induced cognitive impairment and oxidative stress in rats. Nutr Neurosci. 2015; 18:210–216. PMID: 24559058.22. Atcha Z, Rourke C, Neo AH, Goh CW, Lim JS, Aw CC, Browne ER, Pemberton DJ. Alternative method of oral dosing for rats. J Am Assoc Lab Anim Sci. 2010; 49:335–343. PMID: 20587166.23. Van Pelt LF. Ketamine and xylazine for surgical anesthesia in rats. J Am Vet Med Assoc. 1977; 171:842–844. PMID: 924855.24. Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984; 21:130–132. PMID: 6490072.25. Wasowicz W, Neve J, Peretz A. Optimized steps in fluorometric determination of thiobarbituric acid-reactive substances in serum: importance of extraction pH and influence of sample preservation and storage. Clin Chem. 1993; 39:2522–2526. PMID: 8252725.26. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967; 70:158–169. PMID: 6066618.27. Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972; 47:389–394. PMID: 4556490.28. Peralta C, Hotter G, Closa D, Prats N, Xaus C, Gelpí E, Roselló-Catafau J. The protective role of adenosine in inducing nitric oxide synthesis in rat liver ischemia preconditioning is mediated by activation of adenosine A2 receptors. Hepatology. 1999; 29:126–132. PMID: 9862858.29. Hortelano S, Genaro AM, Bosca L. Phorbol esters induce nitric oxide synthase activity in rat hepatocytes. Antagonism with the induction elicited by lipopolysaccharide. J Biol Chem. 1992; 267:24937–24940. PMID: 1281151.30. French SW, Miyamoto K, Ohta Y, Geoffrion Y. Pathogenesis of experimental alcoholic liver disease in the rat. Methods Achiev Exp Pathol. 1988; 13:181–207. PMID: 3045495.31. Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nuñez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G. Nomenclature Committee on Cell Death 2009. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009; 16:3–11. PMID: 18846107.32. Strubelt O, Younes M, Pentz R, Kuhnel W. Mechanistic study on formaldehyde-induced hepatotoxicity. J Toxicol Environ Health. 1989; 27:351–366. PMID: 2754759.33. Nasiri E, Naserirad S, Pasdaran Lashgari A, Gazor R, Mohammadghasemi F, Atrkar Roushan Z. Hepatoprotective effect of Acantholimon bracteatum (Girard) Boiss. on formaldehyde-induced liver injury in adult male mice. Res J Pharmacogn. 2016; 3:55–61.34. Lovschall H, Eiskjaer M, Arenholt-Bindslev D. Formaldehyde cytotoxicity in three human cell types assessed in three different assays. Toxicol In Vitro. 2002; 16:63–69. PMID: 11812641.35. Saito Y, Nishio K, Yoshida Y, Niki E. Cytotoxic effect of formaldehyde with free radicals via increment of cellular reactive oxygen species. Toxicology. 2005; 210:235–245. PMID: 15840437.36. Ghosh N, Ghosh R, Mandal V, Mandal SC. Recent advances in herbal medicine for treatment of liver diseases. Pharm Biol. 2011; 49:970–988. PMID: 21595500.37. Kolios G, Valatas V, Kouroumalis E. Role of Kupffer cells in the pathogenesis of liver disease. World J Gastroenterol. 2006; 12:7413–7420. PMID: 17167827.38. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Elsevier;2007.39. Gerin F, Erman H, Erboga M, Sener U, Yilmaz A, Seyhan H, Gurel A. The effects of ferulic acid against oxidative stress and inflammation in formaldehyde-induced hepatotoxicity. Inflammation. 2016; 39:1377–1386. PMID: 27235018.40. Yang MS, Chan HW, Yu LC. Glutathione peroxidase and glutathione reductase activities are partially responsible for determining the susceptibility of cells to oxidative stress. Toxicology. 2006; 226:126–130. PMID: 16887253.41. Bakar E, Ulucam E, Cerkezkayabekir A. Protective effects of proanthocyanidin and vitamin E against toxic effects of formaldehyde in kidney tissue. Biotech Histochem. 2015; 90:69–78. PMID: 25225844.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of administration of etretinate and fish oil on plasma cholesterol levels in rats
- Ameliorative effects of type-A procyanidins polyphenols from cinnamon bark in compound 48/80-induced mast cell degranulation
- Protective effects of pine bark extract against cisplatin-induced hepatotoxicity and oxidative stress in rats
- A Case of Risperidone-Induced Hepatotoxicity
- Protective effects of Artemisia arborescens essential oil on oestroprogestative treatment induced hepatotoxicity