Nutr Res Pract.
2015 Oct;9(5):466-471. 10.4162/nrp.2015.9.5.466.
Protective effects of Artemisia arborescens essential oil on oestroprogestative treatment induced hepatotoxicity
- Affiliations
-
- 1Physiopathologie environnementale, valorisation des molecules bioactives et modelisation mathematique, Faculty of Sciences of Sfax, Road Soukra km 3.5 - PB n(degrees) 1171-3000 Sfax-, Tunisia. s.dhibi@yahoo.fr
- KMID: 2313866
- DOI: http://doi.org/10.4162/nrp.2015.9.5.466
Abstract
- BACKGROUND
Currently, natural products have been shown to exhibit interesting biological and pharmacological activities and are used as chemotherapeutic agents. The purpose of this study, conducted on Wistar rats, was to evaluate the beneficial effects of Artemisia arborescens oil on oestroprogestative treatment induced damage on liver.
MATERIALS/METHODS
A total of 36 Wistar rats were divided into 4 groups; a control group (n = 9), a group of rats who received oestroprogestative treatment by intraperitoneal injection (n = 9), a group pre-treated with Artemisia arborescens then injected with oestroprogestative treatment (n = 9), and a group pre-treated with Artemisia arborescens (n = 9). To minimize the handling stress, animals from each group were sacrificed rapidly by decapitation. Blood serum was obtained by centrifugation and the livers were removed, cleaned of fat, and stored at -80degrees C until use.
RESULTS
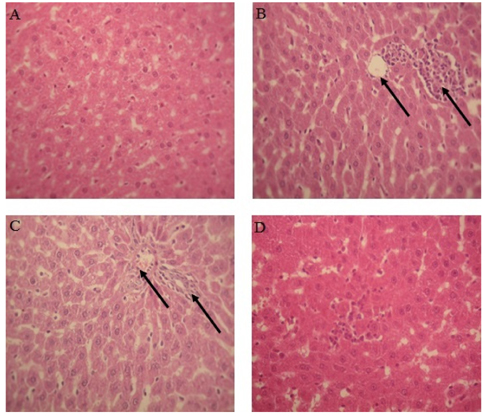
In the current study, oestroprogestative poisoning resulted in oxidative stress, which was demonstrated by 1) a significant increase of lipid peroxidation level in hepatic tissue 2) increased levels of serum transaminases (aspartate amino transferase and serum alanine amino transferase), alkaline phosphatase, glycemia and triglycerides and a decrease in the level of cholesterol 3) alteration of hepatic architecture. Pre-administration of Artemisia arborescens oil was found to alleviate oestroprogestative treatment induced damage by lowering lipid peroxidation level and by increasing activity of catalase, superoxide-dismutase, and glutathione-peroxidase in liver and by reducing disruption of biochemical parameters.
CONCLUSION
Therefore, the results obtained in this study confirmed that Artemisia essential oil protects against oestroprogestative administration induced hepatotoxicity by restoration of liver activities.
MeSH Terms
-
Alanine
Alkaline Phosphatase
Animals
Artemisia*
Biological Products
Catalase
Centrifugation
Cholesterol
Decapitation
Injections, Intraperitoneal
Lipid Peroxidation
Liver
Oxidative Stress
Poisoning
Rats
Rats, Wistar
Serum
Transaminases
Transferases
Triglycerides
Alanine
Alkaline Phosphatase
Biological Products
Catalase
Cholesterol
Transaminases
Transferases
Triglycerides
Figure
Reference
-
1. Mladenović D, Radosavljević T, Ninković M, Vucević D, Jesić-Vukićević R, Todorović V. Liver antioxidant capacity in the early phase of acute paracetamol-induced liver injury in mice. Food Chem Toxicol. 2009; 47:866–870.
Article2. Potts RO, Lobo RA. Transdermal drug delivery: clinical considerations for the obstetrician-gynecologist. Obstet Gynecol. 2005; 105:953–961.
Article3. Elouni B, Ben Salem C, Zamy M, Ganne N, Beaugrand M, Bouraoui K, Biour M. Cytolytic hepatitis possibly related to levonorgestrel/ethinylestradiol oral contraceptive use: 2 case reports. Ann Pharmacother. 2010; 44:2035–2037.
Article4. Huang L, Smit JW, Meijer DK, Vore M. Mrp2 is essential for estradiol-17beta(beta-D-glucuronide)-induced cholestasis in rats. Hepatology. 2000; 32:66–72.
Article5. Sitruk-Ware RL, Menard J, Rad M, Burggraaf J, de Kam ML, Tokay BA, Sivin I, Kluft C. Comparison of the impact of vaginal and oral administration of combined hormonal contraceptives on hepatic proteins sensitive to estrogen. Contraception. 2007; 75:430–437.
Article6. Goldfarb S. Sex hormones and hepatic neoplasia. Cancer Res. 1976; 36:2584–2588.7. Rezaeinodehi A, Khangholi S. Chemical composition of the essential oil of Artemisia absinthium growing wild in Iran. Pak J Biol Sci. 2008; 11:946–949.
Article8. Ghasemi K, Ghasemi Y, Ebrahimzadeh MA. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak J Pharm Sci. 2009; 22:277–281.9. Lee HW, Ko YH, Lim SB. Effects of selected plant extracts on anti-oxidative enzyme activities in rats. Food Chem. 2012; 132:1276–1280.
Article10. Kordali S, Cakir A, Mavi A, Kilic H, Yildirim A. Screening of chemical composition and antifungal and antioxidant activities of the essential oils from three Turkish artemisia species. J Agric Food Chem. 2005; 53:1408–1416.
Article11. Presti ML, Crupi ML, Zellner BD, Dugo G, Mondello L, Dugo P, Ragusa S. Characterization of Artemisia arborescens L. (Asteraceae) leaf-derived essential oil from Southern Italy. J Essent Oil Res. 2007; 19:218–224.
Article12. Sharopov FS, Sulaimonova VA, Setzer WN. Composition of the essential oil of Artemisia absinthium from Tajikistan. Rec Nat Prod. 2012; 6:127–134.13. Lopes-Lutz D, Alviano DS, Alviano CS, Kolodziejczyk PP. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry. 2008; 69:1732–1738.
Article14. Ghasemi Pirbalouti A, Firoznezhad M, Craker L, Akbarzadeh M. Essential oil compositions, antibacterial and antioxidant activities of various populations of Artemisia chamaemelifolia at two phenological stages. Rev Bras Farmacogn. 2013; 23:861–869.
Article15. Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med. 1976; 15:212–216.
Article16. Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988; 34:497–500.
Article17. Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984; 105:114–121.18. Aebi H. Catalase in vitro. Methods Enzymol. 1984; 105:121–126.19. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193:265–275.
Article20. Gabe M. Techniques Histologiques. Paris: Masson et Cie;1968.21. Cay M, Ulas M. Effects of estrogen replacement therapy with vitamin E on oxidative stress in hepatic and pancreatic tissues of ovariectomized diabetic rats. J Anim Vet Adv. 2010; 9:2955–2962.
Article22. Yamamoto Y, Moore R, Hess HA, Guo GL, Gonzalez FJ, Korach KS, Maronpot RR, Negishi M. Estrogen receptor alpha mediates 17alpha-ethynylestradiol causing hepatotoxicity. J Biol Chem. 2006; 281:16625–16631.23. Sabers A. Pharmacokinetic interactions between contraceptives and antiepileptic drugs. Seizure. 2008; 17:141–144.
Article24. Cavalieri EL, Rogan EG, Chakravarti D. Initiation of cancer and other diseases by catechol ortho-quinones: a unifying mechanism. Cell Mol Life Sci. 2002; 59:665–681.
Article25. O'Brien MD, Guillebaud J. Contraception for women taking antiepileptic drugs. J Fam Plann Reprod Health Care. 2010; 36:239–242.26. Akash C, Kankanala VV, Ajit K, Nayana J, Naval C. Cytolytic hepatitis: a rare complication of oral contraceptives. J Clin Diagn Res. 2011; 5:1450–1451.27. Judzentiene A, Tomi F, Casanova J. Analysis of essential oils of Artemisia absinthium L. from Lithuania by CC, GC(RI), GC-MS and 13C NMR. Nat Prod Commun. 2009; 4:1113–1118.
Article28. Lamharrar A, Idlimam A, Ethmane Kane CS, Jamali A, Abdenouri N, Kouhila M. Sorption isotherms and drying characteristics of Artemisia arborescens leaves. J Agron. 2007; 6:488–498.
Article29. Williams D. Contraception and prenatal vitamin supplementation for women on antiepileptic medications. Ment Health Clin. 2013; 3:88–95.
Article30. Abderrahim A, Belhamel K, Chalchat JC, Figuérédo G. Chemical composition of the essential oil from Artemisia arborescens L. growing wild in algeria. Rec Nat Prod. 2010; 4:87–90.31. Mahmoudi M, Ebrahimzadeh MA, Ansaroudi F, Nabavi SF, Nabavi SM. Antidepressant and antioxidant activities of Artemisia absinthium L. at flowering stage. Afr J Biotechnol. 2009; 8:7170–7175.32. Lai F, Loy G, Manconi M, Manca ML, Fadda AM. Artemisia arborescens L essential oil loaded beads: preparation and characterization. AAPS PharmSciTech. 2007; 8:E67.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemical Composition and Antibacterial Activity Against Oral Bacteria by the Essential Oil of Artemisia iwayomogi
- What is In-Jin-Sook? Artemisia capillaris, Artemisia iwayomogi, and Artemisia annua
- Protective effects of pine bark extract against cisplatin-induced hepatotoxicity and oxidative stress in rats
- The Protective Effects of Garlic against Carbon tetrachloride-induced Hepatotoxicity
- Differences in Neurotransmitters Level as Biomarker on Sleep Effects in Dementia Patients with Insomnia after Essential Oils Treatment