J Breast Cancer.
2019 Sep;22(3):362-374. 10.4048/jbc.2019.22.e34.
Anticancer Activity of Tubulosine through Suppression of Interleukin-6-Induced Janus Kinase 2/Signal Transducer and Activation of Transcription 3 Signaling
- Affiliations
-
- 1Department of Pharmacology, Seoul National University College of Medicine, Seoul, Korea. sangkyu@snu.ac.kr
- 2Biomedical Science Project (BK21 PLUS), Seoul National University College of Medicine, Seoul, Korea.
- 3Ischemic/Hypoxic Disease Institute, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Oriental Rehabilitation Medicine, College of Oriental Medicine, Daejeon University, Daejeon, Korea.
- 5Division of Basic Radiation Bioscience, Korea Institute of Radiological and Medical Sciences, Seoul, Korea.
- 6Department of Applied Chemistry, Daejeon University, Daejeon, Korea.
- 7Neuro-Immune Information Storage Network Research Center, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2458858
- DOI: http://doi.org/10.4048/jbc.2019.22.e34
Abstract
- PURPOSE
The chemical structure of tubulosine has been known since the mid-1960s. However, little is known about its biological and pharmacological functions. The aim of this study was to investigate the novel functions of tubulosine in cancer treatment, specifically in breast cancer.
METHODS
An Unpaired (Upd)-induced Drosophila cell line and interleukin (IL)-6-stimulated human breast cancer cell lines were used to investigate the biological and pharmacological activities of tubulosine in vitro. To investigate the activities of tubulosine, we performed molecular and cellular experiments such as Western blot and reverse transcription polymerase chain reaction analyses, immunoprecipitation and terminal deoxynucleotidyl transferase dUTP nick end labeling assays, and immunofluorescence staining using breast cancer cell lines.
RESULTS
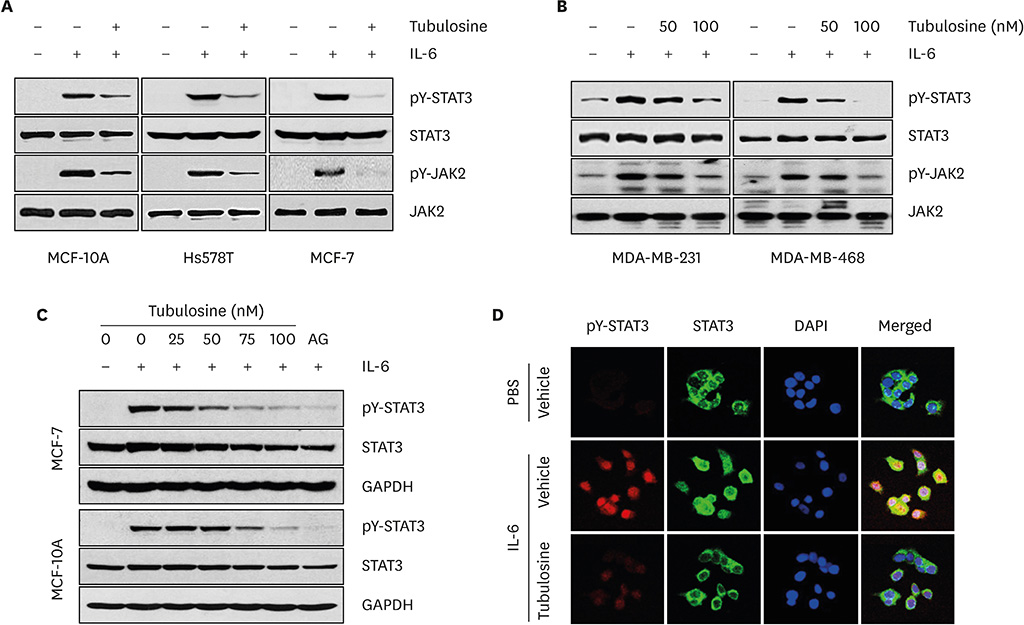
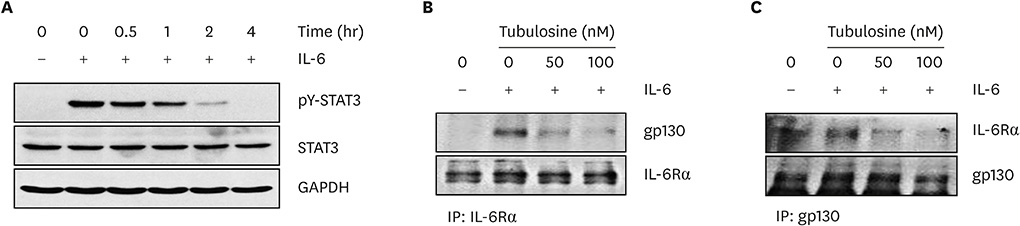
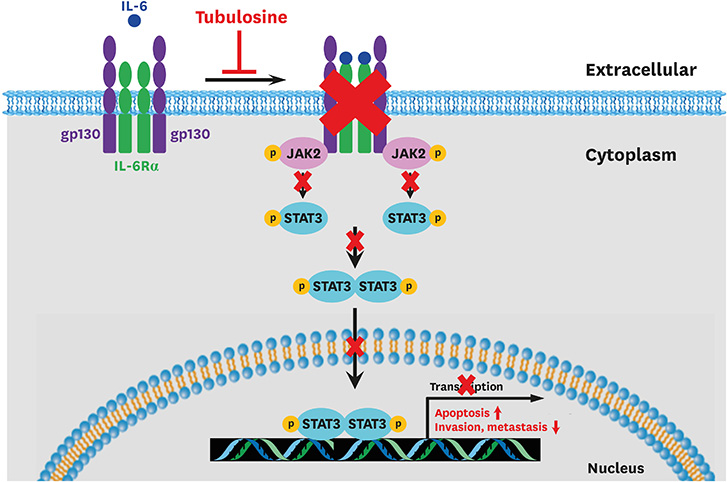
Tubulosine exhibited anticancer activity in IL-6-stimulated human breast cancer cells. Moreover, tubulosine reduced the tyrosine phosphorylation level and transcriptional activity of signal transducer and activator of transcription (STAT) protein at 92E in Upd-induced Drosophila cells. Additionally, tubulosine suppressed IL-6-induced Janus kinase 2 (JAK2)/STAT3 signaling, resulting in decreased viability and induction of apoptotic cell death in breast cancer cells. Interestingly, inhibition of IL-6-induced JAK2/STAT3 signaling by tubulosine was associated with the blocking of IL-6 receptor (IL-6R) and glycoprotein 130 (gp130) binding.
CONCLUSION
Tubulosine exhibits anticancer activity through functional inhibition of IL-6-induced JAK2/STAT3 signaling by targeting IL-6Rα/gp130 binding in breast cancer cells. These findings suggest that tubulosine may hold promise for the treatment of inflammation-associated cancers, including breast cancer.
MeSH Terms
-
Blotting, Western
Breast Neoplasms
Cell Death
Cell Line
DNA Nucleotidylexotransferase
Drosophila
Fluorescent Antibody Technique
Glycoproteins
Humans
Immunoprecipitation
In Vitro Techniques
Interleukin-6
Interleukins
Janus Kinase 2
Phosphorylation
Phosphotransferases*
Polymerase Chain Reaction
Receptors, Interleukin-6
Reverse Transcription
STAT3 Transcription Factor
Transducers*
Tyrosine
DNA Nucleotidylexotransferase
Glycoproteins
Interleukin-6
Interleukins
Janus Kinase 2
Phosphotransferases
Receptors, Interleukin-6
STAT3 Transcription Factor
Tyrosine
Figure
Reference
-
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018; 68:7–30.
Article2. Kuhl CK. The changing world of breast cancer: a radiologist's perspective. Invest Radiol. 2015; 50:615–628.3. Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012; 486:346–352.
Article4. Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016; 13:674–690.
Article5. Schlotter CM, Vogt U, Allgayer H, Brandt B. Molecular targeted therapies for breast cancer treatment. Breast Cancer Res. 2008; 10:211.
Article6. Morris ZS, Harari PM. Interaction of radiation therapy with molecular targeted agents. J Clin Oncol. 2014; 32:2886–2893.
Article7. Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012; 366:109–119.
Article8. Yang G, Nowsheen S, Aziz K, Georgakilas AG. Toxicity and adverse effects of Tamoxifen and other anti-estrogen drugs. Pharmacol Ther. 2013; 139:392–404.
Article9. Marotta LL, Almendro V, Marusyk A, Shipitsin M, Schemme J, Walker SR, et al. The JAK2/STAT3 signaling pathway is required for growth of CD44+CD24− stem cell-like breast cancer cells in human tumors. J Clin Invest. 2011; 121:2723–2735.
Article10. Kim BH, Yi EH, Ye SK. Signal transducer and activator of transcription 3 as a therapeutic target for cancer and the tumor microenvironment. Arch Pharm Res. 2016; 39:1085–1099.
Article11. Brauchli P, Deulofeu V, Budzikiewicz H, Djerassi C. The structure of tubulosine, a novel alkaloid from Pogonopus tubulosus (DC.) Schumann. J Am Chem Soc. 1964; 86:1895–1896.
Article12. Kim BH, Yin CH, Guo Q, Bach EA, Lee H, Sandoval C, et al. A small-molecule compound identified through a cell-based screening inhibits JAK/STAT pathway signaling in human cancer cells. Mol Cancer Ther. 2008; 7:2672–2680.
Article13. Arbouzova NI, Zeidler MP. JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development. 2006; 133:2605–2616.
Article14. Johnson DE, O'Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018; 15:234–248.
Article15. Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond). 2012; 122:143–159.
Article16. Heo TH, Wahler J, Suh N. Potential therapeutic implications of IL-6/IL-6R/gp130-targeting agents in breast cancer. Oncotarget. 2016; 7:15460–15473.
Article17. Meydan N, Grunberger T, Dadi H, Shahar M, Arpaia E, Lapidot Z, et al. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature. 1996; 379:645–648.
Article18. Decker P, Isenberg D, Muller S. Inhibition of caspase-3-mediated poly(ADP-ribose) polymerase (PARP) apoptotic cleavage by human PARP autoantibodies and effect on cells undergoing apoptosis. J Biol Chem. 2000; 275:9043–9046.
Article19. Bartsch JE, Staren ED, Appert HE. Matrix metalloproteinase expression in breast cancer. J Surg Res. 2003; 110:383–392.
Article20. Zhang BN, Cao XC, Chen JY, Chen J, Fu L, Hu XC, et al. Guidelines on the diagnosis and treatment of breast cancer (2011 edition). Gland Surg. 2012; 1:39–61.21. Won C, Kim BH, Yi EH, Choi KJ, Kim EK, Jeong JM, et al. Signal transducer and activator of transcription 3-mediated CD133 up-regulation contributes to promotion of hepatocellular carcinoma. Hepatology. 2015; 62:1160–1173.
Article22. Hartman ZC, Poage GM, den Hollander P, Tsimelzon A, Hill J, Panupinthu N, et al. Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer Res. 2013; 73:3470–3480.
Article23. Boulanger MJ, Chow DC, Brevnova EE, Garcia KC. Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science. 2003; 300:2101–2104.
Article24. Hong SS, Choi JH, Lee SY, Park YH, Park KY, Lee JY, et al. A Novel small-molecule inhibitor targeting the IL-6 receptor β subunit, glycoprotein 130. J Immunol. 2015; 195:237–245.
Article25. Ma WW, Anderson JE, McKenzie AT, Byrn SR, McLaughlin JL, Hudson MS. Tubulosine: an antitumor constituent of Pogonopus speciosus . J Nat Prod. 1990; 53:1009–1014.26. Ito A, Lee YH, Chai HB, Gupta MP, Farnsworth NR, Cordell GA, et al. 1′,2′,3′,4′-tetradehydrotubulosine, a cytotoxic alkaloid from Pogonopus speciosus . J Nat Prod. 1999; 62:1346–1348.
Article27. Grollman AP. Structural basis for the inhibition of protein biosynthesis: mode of action of tubulosine. Science. 1967; 157:84–85.
Article28. Carrasco L, Jimenez A, Vázquez D. Specific inhibition of translocation by tubulosine in eukaryotic polysomes. Eur J Biochem. 1976; 64:1–5.
Article29. Klausmeyer P, McCloud TG, Uranchimeg B, Melillo G, Scudiero DA, Cardellina JH 2nd, et al. Separation and SAR study of HIF-1alpha inhibitory tubulosines from Alangium cf. longiflorum. Planta Med. 2008; 74:258–263.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Janus Kinase 2 Inhibitor AG490 Inhibits the STAT3 Signaling Pathway by Suppressing Protein Translation of gp130
- Jak1/Stat3 Is an Upstream Signaling of NF-kappaB Activation in Helicobacter pylori-Induced IL-8 Production in Gastric Epithelial AGS Cells
- Role of STAT3 Phosphorylation in Ethanol-Mediated Proliferation of Breast Cancer Cells
- Glutamine Deprivation Causes Hydrogen Peroxide-induced Interleukin-8 Expression via Jak1/Stat3 Activation in Gastric Epithelial AGS Cells
- Thyrotropin Suppresses INF-r Mediated Gene Expression by Inhibiting Signal Transducer and Activation of Transcription 1(STAT1) Activity in FRTL-5 Cells