J Breast Cancer.
2016 Jun;19(2):122-132. 10.4048/jbc.2016.19.2.122.
Role of STAT3 Phosphorylation in Ethanol-Mediated Proliferation of Breast Cancer Cells
- Affiliations
-
- 1Department of Biotechnology, Anna University, Chennai, India. lakshmibs@annauniv.edu
- 2Department of Biochemistry, University of Madras, Chennai, India.
- 3Centre for Food Technology, Department of Biotechnology, Anna University, Chennai, India.
- KMID: 2308962
- DOI: http://doi.org/10.4048/jbc.2016.19.2.122
Abstract
- PURPOSE
In this study, we investigated the molecular mechanism involved in ethanol (EtOH)-mediated proliferation of breast cancer cells.
METHODS
EtOH concentration was optimized by studying its effect on cell proliferation in MCF-7 and MDA MB-231 cells. We used flow cytometry and immunoblot analysis to evaluate the increased proliferation caused by the optimized concentrations of EtOH. The mechanism of EtOH-mediated proliferation was determined using reactive oxygen species (ROS) release assay, reverse transcription polymerase chain reaction, and immunoblot studies. Gene silencing followed by quantitative real-time polymerase chain reaction studies and inhibitor studies indicated the involvement of signal transducer and activator of transcription 3 (STAT3) in EtOH-mediated breast cancer proliferation.
RESULTS
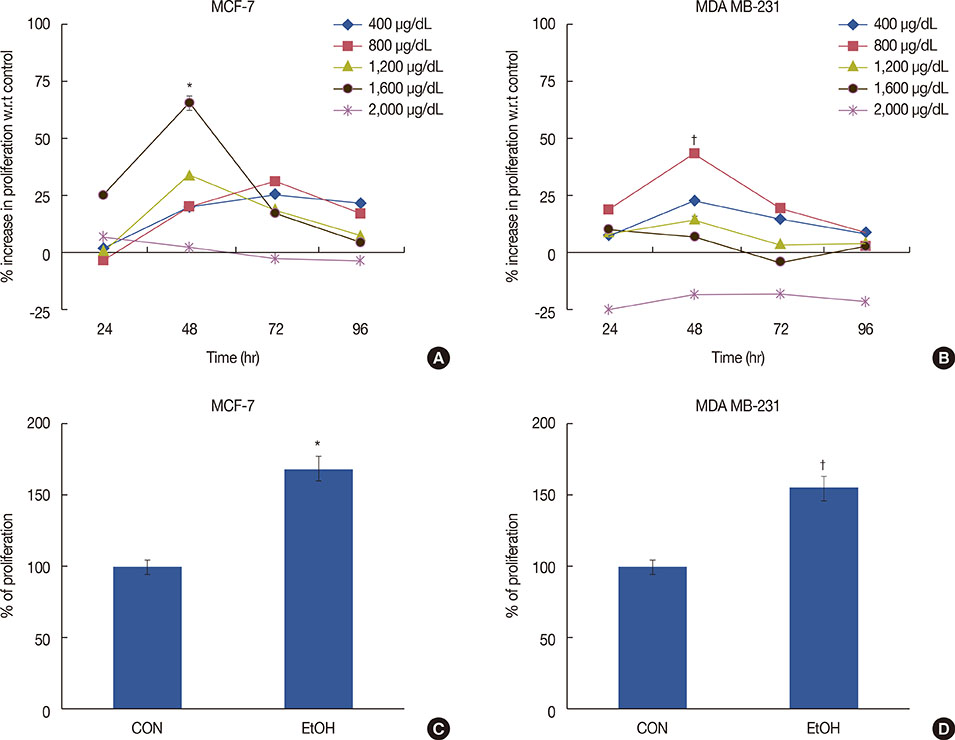
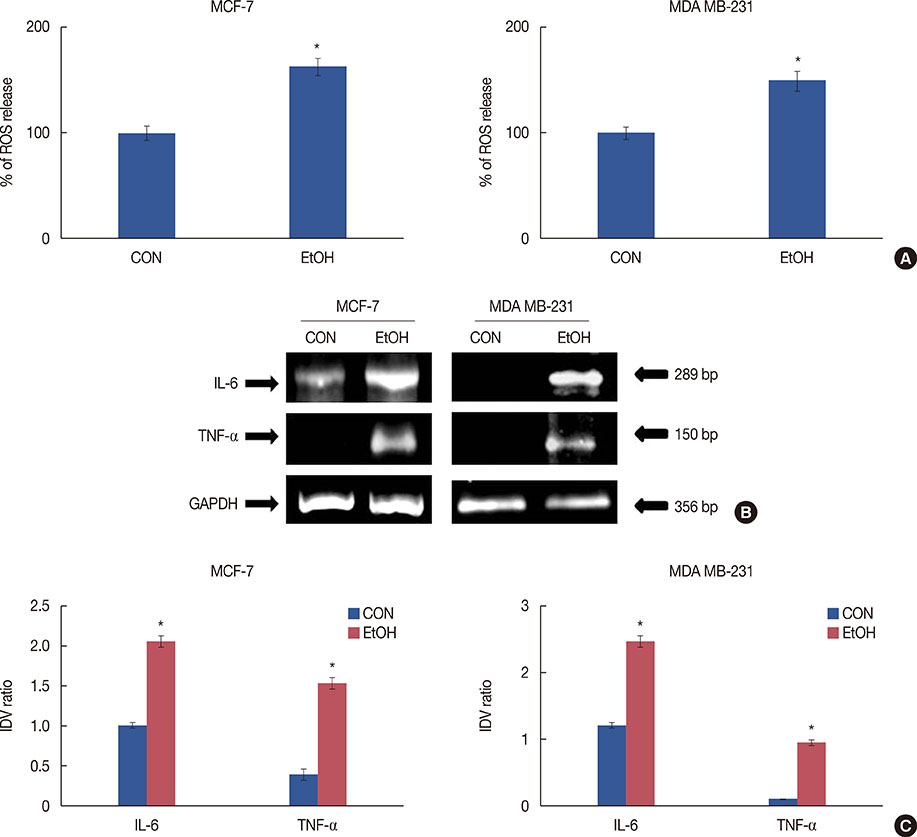
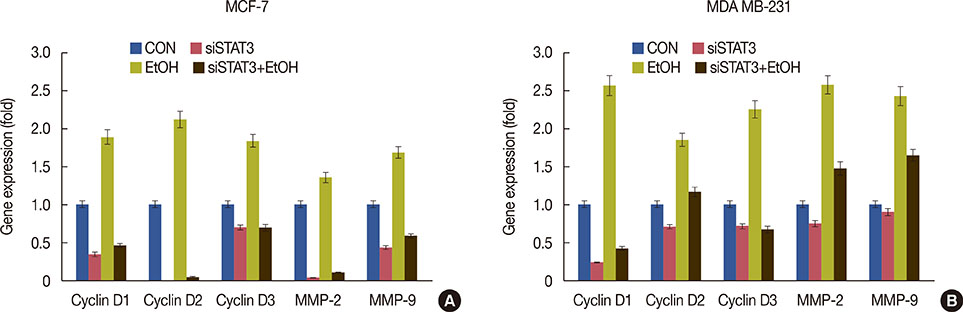
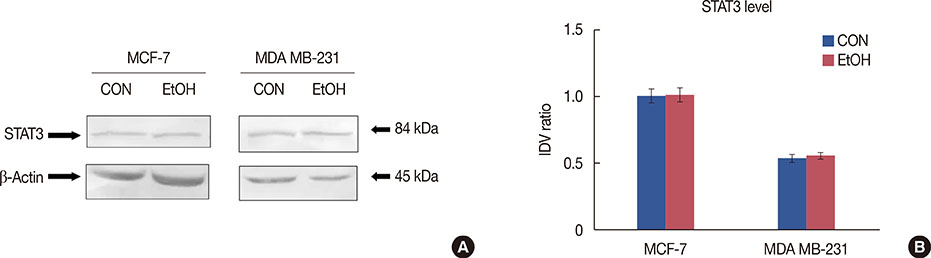
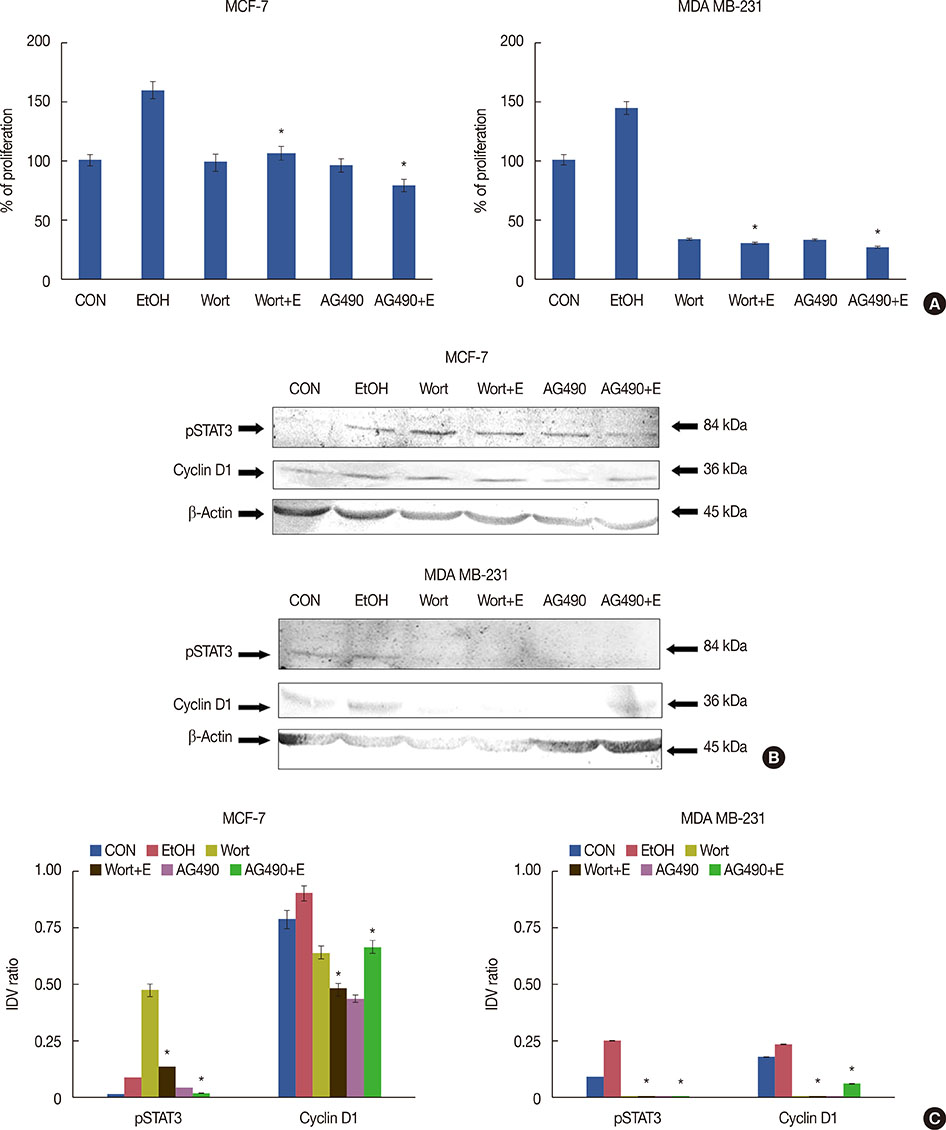
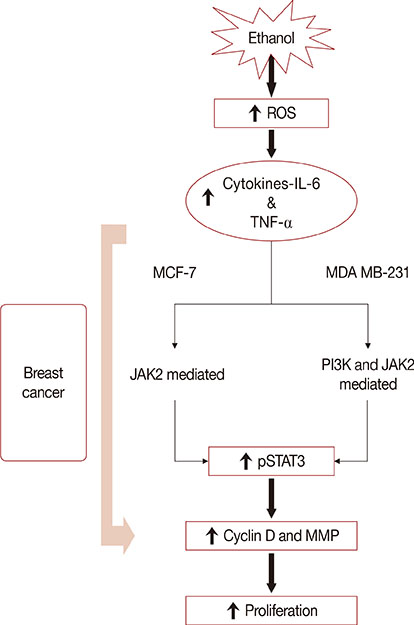
Exposure to EtOH caused an increase in cell proliferation and an accumulation of cells in S-phase in MCF-7 (347 µM EtOH) and MDA MB-231 (173 µM EtOH) cells. Additionally, increased release of ROS and the expression of pro-inflammatory cytokines, such as interleukin 6 and tumor necrosis factor α, confirmed that the proliferation was induced by the ROS-linked inflammatory response in breast cancer. The proinflammatory response was followed by phosphorylation of STAT3. The importance of STAT3 activation in EtOH-mediated proliferation was confirmed through the silencing of STAT3, followed by an investigation on the expression of cyclins and matrix metalloproteinases. Finally, studies using specific inhibitors indicated that the EtOH-mediated effect on STAT3 activation could be regulated by phosphoinositide-3-kinase and Janus kinase 2.
CONCLUSION
The study demonstrates the involvement of STAT3 signaling in EtOH-mediated breast cancer proliferation.
Keyword
MeSH Terms
-
Breast Neoplasms*
Breast*
Cell Proliferation
Cyclins
Cytokines
Ethanol
Flow Cytometry
Gene Silencing
Inflammation
Interleukin-6
Janus Kinase 2
Matrix Metalloproteinases
Phosphorylation*
Polymerase Chain Reaction
Reactive Oxygen Species
Real-Time Polymerase Chain Reaction
Reverse Transcription
STAT3 Transcription Factor
Tumor Necrosis Factor-alpha
Cyclins
Cytokines
Ethanol
Interleukin-6
Janus Kinase 2
Matrix Metalloproteinases
Reactive Oxygen Species
STAT3 Transcription Factor
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010; 140:883–899.
Article2. Oyesanmi O, Snyder D, Sullivan N, Reston J, Treadwell J, Schoelles KM. Alcohol Consumption and Cancer Risk: Understanding Possible Causal Mechanisms for Breast and Colorectal Cancers. Rockville: Agency for Healthcare Research and Quality, U.S. Dept. of Health and Human Services;2010.3. Zhong Q, Shi G, Zhang Y, Lu L, Levy D, Zhong S. Alteration of BRCA1 expression affects alcohol-induced transcription of RNA Pol III-dependent genes. Gene. 2015; 556:74–79.
Article4. Xu M, Bower KA, Wang S, Frank JA, Chen G, Ding M, et al. Cyanidin-3-glucoside inhibits ethanol-induced invasion of breast cancer cells overexpressing ErbB2. Mol Cancer. 2010; 9:285.
Article5. Wang L, Son YO, Ding S, Wang X, Hitron JA, Budhraja A, et al. Ethanol enhances tumor angiogenesis in vitro induced by low-dose arsenic in colon cancer cells through hypoxia-inducible factor 1 alpha pathway. Toxicol Sci. 2012; 130:269–280.
Article6. Xu M, Chen G, Fu W, Liao M, Frank JA, Bower KA, et al. Ethanol disrupts vascular endothelial barrier: implication in cancer metastasis. Toxicol Sci. 2012; 127:42–53.
Article7. Goldberg JE, Schwertfeger KL. Proinflammatory cytokines in breast cancer: mechanisms of action and potential targets for therapeutics. Curr Drug Targets. 2010; 11:1133–1146.
Article8. Carballo M, Conde M, El Bekay R, Martín-Nieto J, Camacho MJ, Monteseirín J, et al. Oxidative stress triggers STAT3 tyrosine phosphorylation and nuclear translocation in human lymphocytes. J Biol Chem. 1999; 274:17580–17586.
Article9. Xiong A, Yang Z, Shen Y, Zhou J, Shen Q. Transcription factor STAT3 as a novel molecular target for cancer prevention. Cancers (Basel). 2014; 6:926–957.
Article10. Posa JK, Selvaraj S, Sangeetha KN, Baskaran SK, Lakshmi BS. p53 mediates impaired insulin signaling in 3T3-L1 adipocytes during hyperinsulinemia. Cell Biol Int. 2014; 38:818–824.
Article11. Sangeetha KN, Sujatha S, Muthusamy VS, Anand S, Nithya N, Velmurugan D, et al. 3Beta-taraxerol of Mangifera indica, a PI3K dependent dual activator of glucose transport and glycogen synthesis in 3T3-L1 adipocytes. Biochim Biophys Acta. 2010; 1800:359–366.
Article12. Shilpa K, Sangeetha KN, Muthusamy VS, Sujatha S, Lakshmi BS. Probing key targets in insulin signaling and adipogenesis using a methanolic extract of Costus pictus and its bioactive molecule, methyl tetracosanoate. Biotechnol Lett. 2009; 31:1837–1841.
Article13. Wong AW, Paulson QX, Hong J, Stubbins RE, Poh K, Schrader E, et al. Alcohol promotes breast cancer cell invasion by regulating the Nm23-ITGA5 pathway. J Exp Clin Cancer Res. 2011; 30:75.
Article14. Bishop JL, Thaper D, Zoubeidi A. The multifaceted roles of STAT3 signaling in the progression of prostate cancer. Cancers (Basel). 2014; 6:829–859.
Article15. Hong J, Holcomb VB, Tekle SA, Fan B, Núñez NP. Alcohol consumption promotes mammary tumor growth and insulin sensitivity. Cancer Lett. 2010; 294:229–235.
Article16. Seitz HK. Alcohol and breast cancer. Breast. 2012; 21:426–427.
Article17. Wagner F, Fink R, Hart R, Lersch C, Dancygier H, Classen M. Ethanol inhibits interferon-gamma secretion by human peripheral lymphocytes. J Stud Alcohol. 1992; 53:277–280.
Article18. Long XE, Gong ZH, Pan L, Zhong ZW, Le YP, Liu Q, et al. Suppression of CDK2 expression by siRNA induces cell cycle arrest and cell proliferation inhibition in human cancer cells. BMB Rep. 2010; 43:291–296.
Article19. Berthet C, Kaldis P. Cdk2 and Cdk4 cooperatively control the expression of Cdc2. Cell Div. 2006; 1:10.20. Wu J, Lv Q, He J, Zhang H, Mei X, Cui K, et al. MicroRNA-188 suppresses G1/S transition by targeting multiple cyclin/CDK complexes. Cell Commun Signal. 2014; 12:66.
Article21. Loayza-Puch F, Drost J, Rooijers K, Lopes R, Elkon R, Agami R. p53 induces transcriptional and translational programs to suppress cell proliferation and growth. Genome Biol. 2013; 14:R32.
Article22. Klampfer L. Cytokines, inflammation and colon cancer. Curr Cancer Drug Targets. 2011; 11:451–464.
Article23. Lin L, Deangelis S, Foust E, Fuchs J, Li C, Li PK, et al. A novel small molecule inhibits STAT3 phosphorylation and DNA binding activity and exhibits potent growth suppressive activity in human cancer cells. Mol Cancer. 2010; 9:217.
Article24. Lian JP, Word B, Taylor S, Hammons GJ, Lyn-Cook BD. Modulation of the constitutive activated STAT3 transcription factor in pancreatic cancer prevention: effects of indole-3-carbinol (I3C) and genistein. Anticancer Res. 2004; 24:133–137.25. Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F, Sawaya R, et al. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene. 2004; 23:3550–3560.
Article26. Wang L, Luo J, He S. Induction of MMP-9 release from human dermal fibroblasts by thrombin: involvement of JAK/STAT3 signaling pathway in MMP-9 release. BMC Cell Biol. 2007; 8:14.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- STAT3 as a Potential Target for Tumor Suppressive Effects of 15-Deoxy-Δ12,14-prostaglandin J2 in Triple Negative Breast Cancer
- Increased Melanoma-Associated Antigen C2 Expression Affords Resistance to Apoptotic Deathin Suspension-Cultured Tumor Cells
- Hydroxyzine Induces Cell Death in Triple-Negative Breast Cancer Cells via Mitochondrial Superoxide and Modulation of Jak2/STAT3 Signaling
- Withaferin-A Inhibits Colon Cancer Cell Growth by Blocking STAT3 Transcriptional Activity
- Isoliquiritigenin Induces Apoptosis via ROS-Mediated Inhibition of p38/mTOR/STAT3 Pathway in Human Melanoma Cells