Korean J Physiol Pharmacol.
2009 Apr;13(2):131-138. 10.4196/kjpp.2009.13.2.131.
Janus Kinase 2 Inhibitor AG490 Inhibits the STAT3 Signaling Pathway by Suppressing Protein Translation of gp130
- Affiliations
-
- 1Department of Physiology, Medical Science Research Institute, College of Medicine, Dong-A University, Busan 602-714, Korea. phwantae@dau.ac.kr
- 2Department of Microbiology, Medical Science Research Institute, College of Medicine, Dong-A University, Busan 602-714, Korea.
- 3Department of Neurology, Medical Science Research Institute, College of Medicine, Dong-A University, Busan 602-714, Korea.
- KMID: 2071662
- DOI: http://doi.org/10.4196/kjpp.2009.13.2.131
Abstract
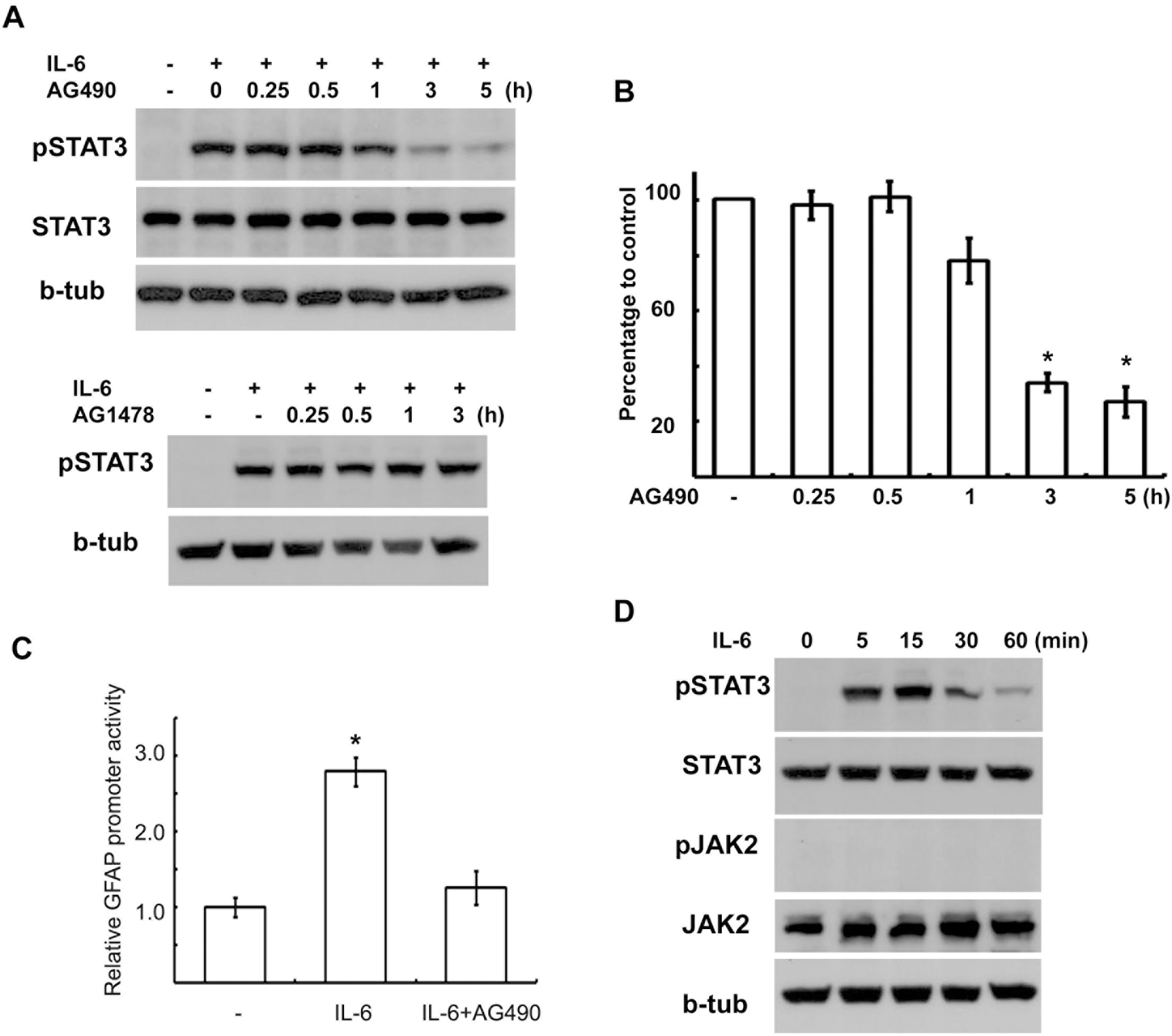
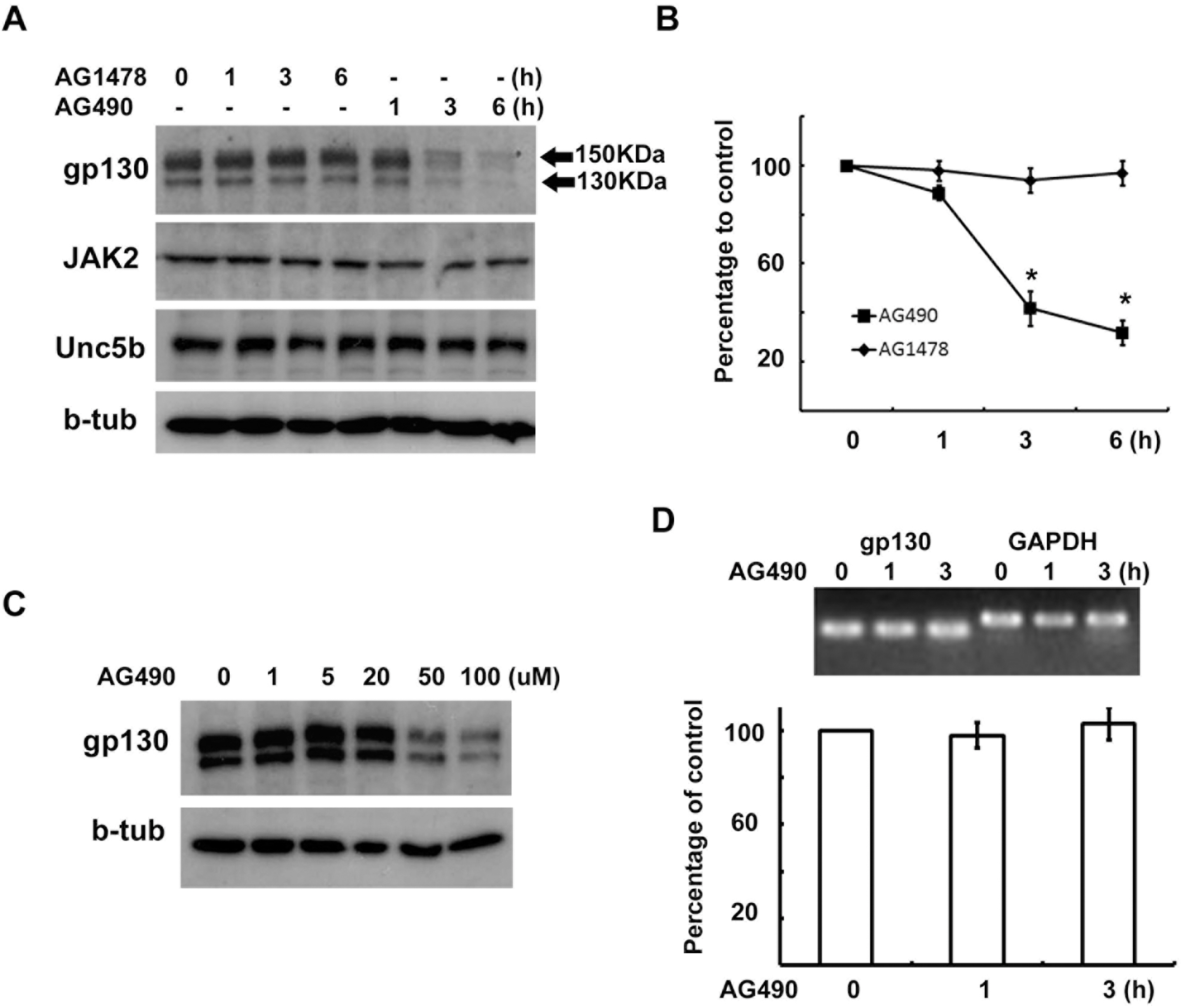
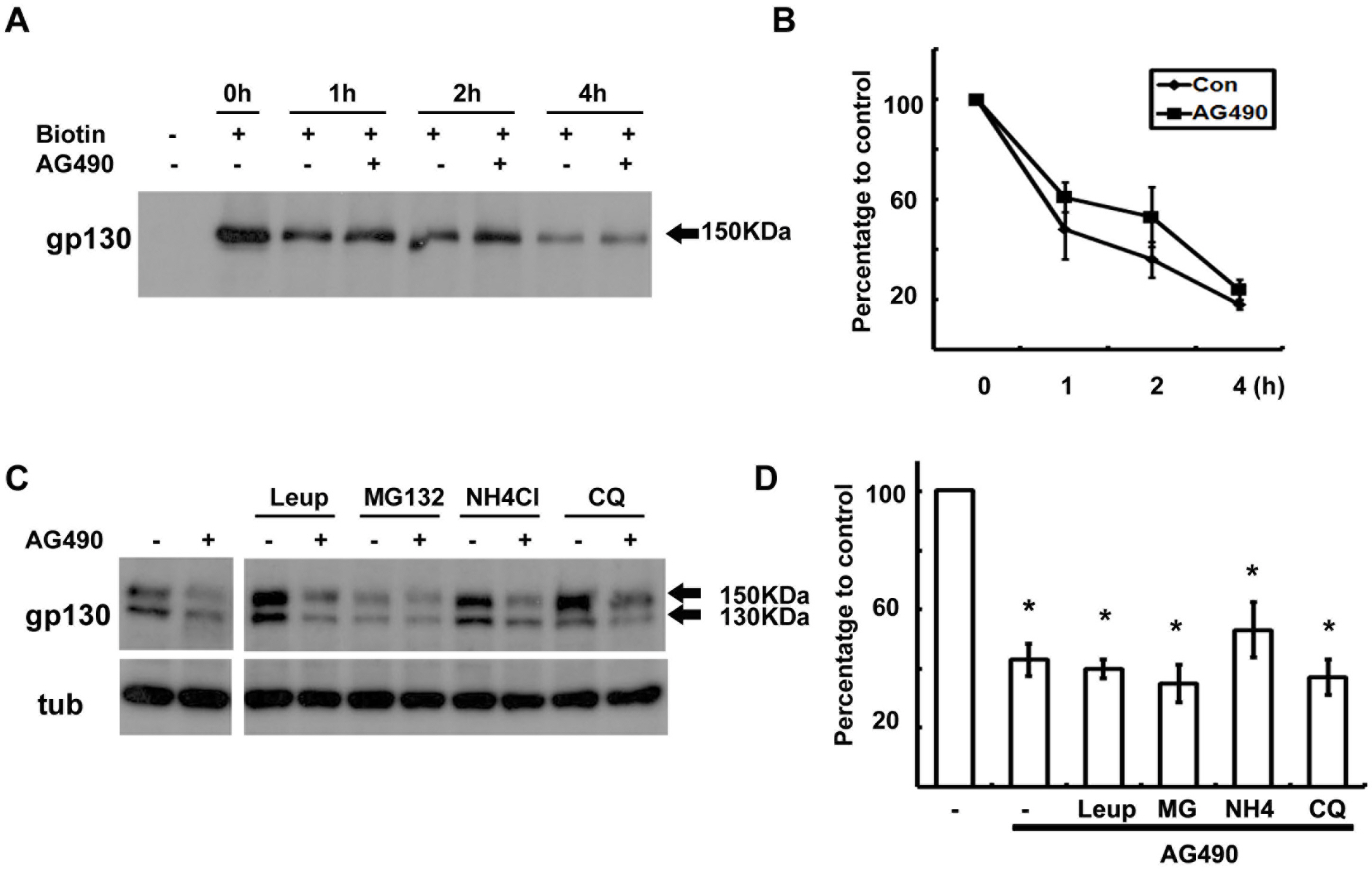
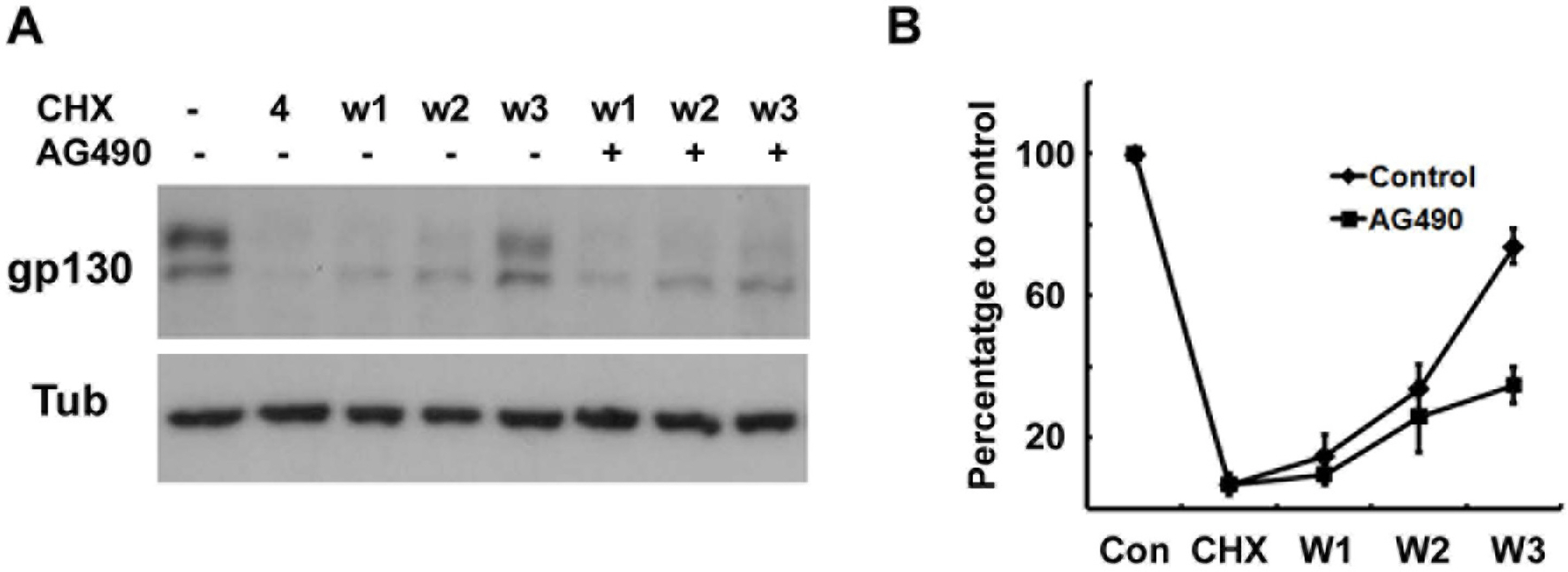
- The binding of interleukin-6 (IL-6) cytokine family ligands to the gp130 receptor complex activates the Janus kinase (JAK)/ signal transducer and activator of transcription 3 (STAT3) signal transduction pathway, where STAT3 plays an important role in cell survival and tumorigenesis. Constitutive activation of STAT3 has been frequently observed in many cancer tissues, and thus, blocking of the gp130 signaling pathway, at the JAK level, might be a useful therapeutic approach for the suppression of STAT3 activity, as anticancer therapy. AG490 is a tyrphostin tyrosine kinase inhibitor that has been extensively used for inhibiting JAK2 in vitro and in vivo. In this study, we demonstrate a novel mechanism associated with AG490 that inhibits the JAK/STAT3 pathway. AG490 induced downregulation of gp130, a common receptor for the IL-6 cytokine family compounds, but not JAK2 or STAT3, within three hours of exposure. The downregulation of gp130 was not caused by enhanced degradation of gp130 or by inhibition of mRNA transcription. It most likely occurred by translation inhibition of gp130 in association with phosphorylation of the eukaryotic initiation factor-2alpha. The inhibition of protein synthesis of gp130 by AG490 led to immediate loss of mature gp130 in cell membranes, due to its short half-life, thereby resulting in reduction in the STAT3 response to IL-6. Taken together, these results suggest that AG490 blocks the STAT3 activation pathway via a novel pathway.
Keyword
MeSH Terms
-
Cell Membrane
Cell Survival
Cell Transformation, Neoplastic
Down-Regulation
Endoplasmic Reticulum Stress
Half-Life
Humans
Interleukin-6
Janus Kinase 2
Ligands
Phosphorylation
Phosphotransferases
Protein Biosynthesis
Protein-Tyrosine Kinases
RNA, Messenger
Signal Transduction
STAT3 Transcription Factor
Tyrphostins
Interleukin-6
Janus Kinase 2
Ligands
Phosphotransferases
Protein-Tyrosine Kinases
RNA, Messenger
STAT3 Transcription Factor
Tyrphostins
Figure
Cited by 1 articles
-
Inhibition of the Interleukin-11-STAT3 Axis Attenuates Hypoxia-Induced Migration and Invasion in MDA-MB-231 Breast Cancer Cells
Ji-Hong Lim
Korean J Physiol Pharmacol. 2014;18(5):391-396. doi: 10.4196/kjpp.2014.18.5.391.
Reference
-
Aggarwal BB., Sethi G., Ahn KS., Sandur SK., Pandey MK., Kunnumakkara AB., Sung B., Ichikawa H. Targeting signal- transducer-and-activator-of-transcription (STAT)-3 for prevention and therapy of cancer: modern target but ancient solution. Ann N Y Acad Sci. 1091:151–169. 2006.Battle TE., Frank DA. The role of STATs in apoptosis. Curr Mol Med. 2:381–392. 2002.
ArticleBertolotti A., Zhang Y., Hendershot LM., Harding HP., Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2:326–332. 2000.
ArticleFerrajoli A., Faderl S., Van Q., Koch P., Harris D., Liu Z., Hazan-Halevy I., Wang Y., Kantarjian HM., Priebe W., Estrov Z. WP1066 disrupts Janus kinase-2 and induces caspase-dependent apoptosis in acute myelogenous leukemia cells. Cancer Res. 67:11291–11299. 2009.
ArticleGerhartz C., Dittrich E., Stoyan T., Rose-John S., Yasukawa K., Heinrich PC., Graeve L. Biosynthesis and half-life of the interleukin-6 receptor and its signal transducer gp130. Eur J Biochem. 223:265–274. 1994.
ArticleGermain D., Frank DA. Targeting the cytoplasmic and nuclear functions of signal transducers and activators of transcription 3 for cancer therapy. Clin Cancer Res. 13:5665–5669. 2007.Graf D., Haselow K., Münks I., Bode JG., Häussinger D. Caspase-mediated cleavage of the signal-transducing IL-6 receptor subunit gp130. Arch Biochem Biophys. 477:330–338. 2008.
ArticleGraf D., Kohlmann C., Haselow K., Gehrmann T., Bode JG., Häussinger D. Bile acids inhibit interleukin-6 signaling via gp130 receptor-dependent and -independent pathways in rat liver. Hepatology. 44:1206–1217. 2006.
ArticleHarding HP., Zhang Y., Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 397:271–274. 1999.
ArticleHeinrich PC., Behrmann I., Haan S., Hermanns HM., Müller-Newen G., Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 374:1–20. 2003.
ArticleHideshima T., Chauhan D., Hayashi T., Akiyama M., Mitsiades N., Mitsiades C., Podar K., Munshi NC., Richardson PG., Anderson KC. Proteasome inhibitor PS-341 abrogates IL-6 triggered signaling cascades via caspase-dependent downregulation of gp130 in multiple myeloma. Oncogene. 22:8386–8393. 2003.
ArticleLauta VM. Interleukin-6 and the network of several cytokines in multiple myeloma: an overview of clinical and experimental data. Cytokine. 16:79–86. 2001.
ArticleLee HK., Seo IA., Suh DJ., Lee HJ., Park HT. A novel mechanism of methylglyoxal cytotoxicity in neuroglial cells. J Neurochem. 108:273–284. 2009a.
ArticleLee HK., Seo IA., Shin YK., Park JW., Suh DJ., Park HT. Capsaicin inhibits the IL-6/STAT3 pathway by depleting intracellular gp130 pools through endoplasmic reticulum stress. Biochem Biophys Res Commun. 382:445–450. 2009c.
ArticleLee HK., Seo IA., Suh DJ., Hong JI., Yoo YH., Park HT. Interleukin-6 is required for the early induction of glial fibrillary acidic protein in Schwann cells during Wallerian degeneration. J Neurochem. 108:776–786. 2009b.
ArticleMeydan N., Grunberger T., Dadi H., Shahar M., Arpaia E., Lapidot Z., Leeder JS., Freedman M., Cohen A., Gazit A., Levitzki A., Roifman CM. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature. 379:645–648. 1996.
ArticleMiyamoto N., Sugita K., Goi K., Inukai T., Lijima K., Tezuka T., Kojika S., Nakamura M., Kagami K., Nakazawa S. The JAK2 inhibitor AG490 predominantly abrogates the growth of human B-precursor leukemic cells with 11q23 translocation or Philadelphia chromosome. Leukemia. 15:1758–1768. 2001.
ArticleOpdam FJ., Kamp M., de Bruijn R., Roos E. Jak kinase activity is required for lymphoma invasion and metastasis. Oncogene. 23:6647–6653. 2004.
ArticleRahaman SO., Harbor PC., Chernova O., Barnett GH., Vogelbaum MA., Haque SJ. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 55:8404–8413. 2002.
ArticleSamanta AK., Lin H., Sun T., Kantarjian H., Arlinghaus RB. Janus kinase 2: a critical target in chronic myelogenous leukemia. Cancer Res. 66:6468–6472. 2006.
ArticleSatriotomo I., Bowen KK., Vemuganti R. JAK2 and STAT3 activation contributes to neuronal damage following transient focal cerebral ischemia. J Neurochem. 98:1353–1368. 2006.
ArticleScholz A., Heinze S., Detjen KM., Peters M., Welzel M., Hauff P., Schirner M., Wiedenmann B., Rosewicz S. Activated signal transducer and activator of transcription 3 (STAT3) supports the malignant phenotype of human pancreatic cancer. Gastroenterology. 125:891–905. 2003.
ArticleShyu WC., Lin SZ., Chiang MF., Chen DC., Su CY., Wang HJ., Liu RS., Tsai CH., Li H. Secretoneurin promotes neuroprotection and neuronal plasticity via the Jak2/Stat3 pathway in murine models of stroke. J Clin Invest. 118:133–148. 2008.
ArticleSzegezdi E., Logue SE., Gorman AM., Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 7:880–885. 2006.
ArticleTanaka Y., Tanaka N., Saeki Y., Tanaka K., Murakami M., Hirano T., Ishii N., Sugamura K. c-Cbl-dependent monoubiquitination and lysosomal degradation of gp130. Mol Cell Biol. 28:4805–4818. 2008.
ArticleTebbutt NC., Giraud AS., Inglese M., Jenkins B., Waring P., Clay FJ., Malki S., Alderman BM., Grail D., Hollande F., Heath JK., Ernst M. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 8:1089–1097. 2002.
ArticleVerstovsek S., Manshouri T., Quintás-Cardama A., Harris D., Cortes J., Giles FJ., Kantarjian H., Priebe W., Estrov Z. WP1066, a novel JAK2 inhibitor, suppresses proliferation and induces apoptosis in erythroid human cells carrying the JAK2 V617F mutation. Clin Cancer Res. 14:788–796. 2008.
ArticleWeissenberger J., Loeffler S., Kappeler A., Kopf M., Lukes A., Afanasieva TA., Aguzzi A., Weis J. IL-6 is required for glioma development in a mouse model. Oncogene. 23:3308–3316. 2004.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Peroxisome Proliferator-activated Receptor-gamma Inhibits the Activation of STAT3 in Cerulein-stimulated Pancreatic Acinar Cells
- Glutamine Deprivation Causes Hydrogen Peroxide-induced Interleukin-8 Expression via Jak1/Stat3 Activation in Gastric Epithelial AGS Cells
- The role of local IL6/JAK2/STAT3 signaling in high glucose–induced podocyte hypertrophy
- Jak1/Stat3 Is an Upstream Signaling of NF-kappaB Activation in Helicobacter pylori-Induced IL-8 Production in Gastric Epithelial AGS Cells
- Tyrphostin ErbB2 Inhibitors AG825 and AG879 Have Non-specific Suppressive Effects on gp130/ STAT3 Signaling