Immune Netw.
2012 Aug;12(4):148-154.
Calcium/Calmodulin-Dependent Protein Kinase is Involved in the Release of High Mobility Group Box 1 Via the Interferon-beta Signaling Pathway
- Affiliations
-
- 1Department of Pathology, Hallym University College of Medicine, Chuncheon 200-702, Korea. kwonik@hallym.ac.kr
Abstract
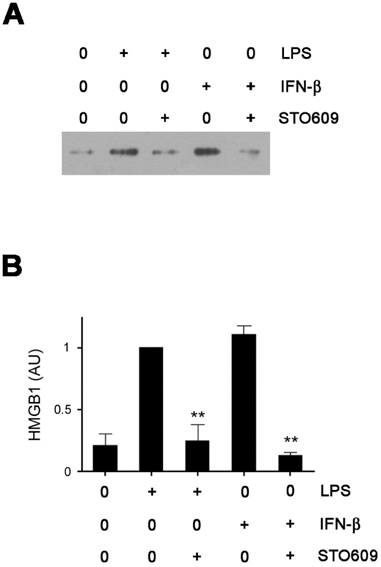
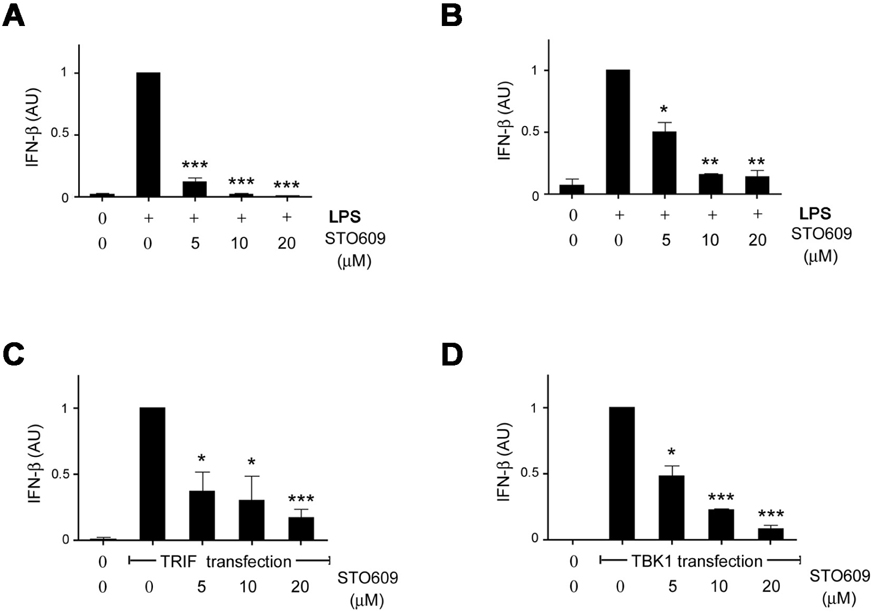
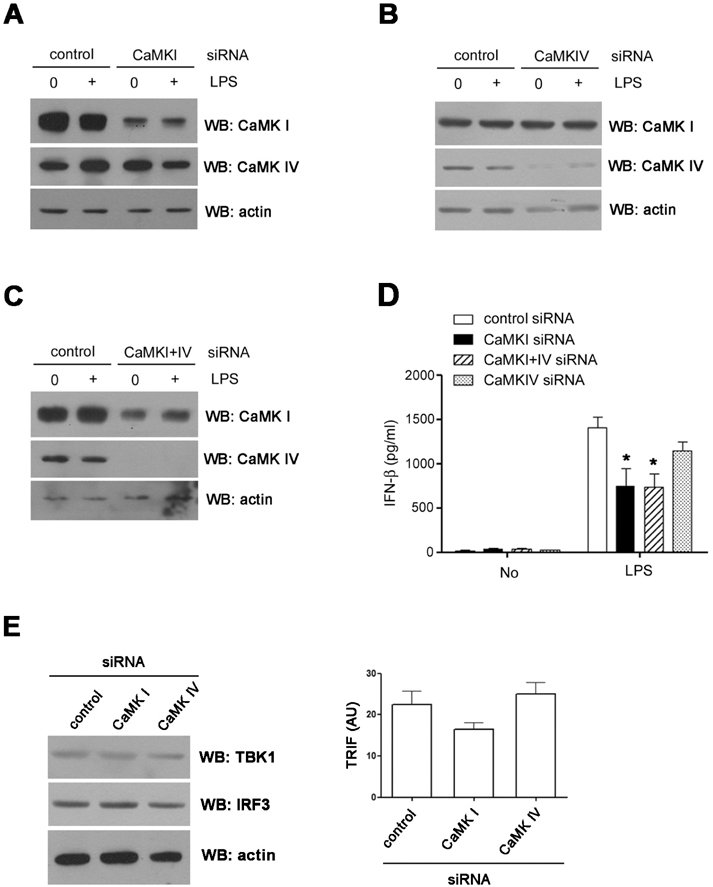
- Previously, we have reported that high mobility group box 1 (HMGB1), a proinflammatory mediator in sepsis, is released via the IFN-beta-mediated JAK/STAT pathway. However, detailed mechanisms are still unclear. In this study, we dissected upstream signaling pathways of HMGB1 release using various molecular biology methods. Here, we found that calcium/calmodulin-dependent protein kinase (CaM kinase, CaMK) is involved in HMGB1 release by regulating IFN-beta production. CaMK inhibitor, STO609, treatment inhibits LPS-induced IFN-beta production, which is correlated with the phosphorylation of interferon regulatory factor 3 (IRF3). Additionally, we show that CaMK-I plays a major role in IFN-beta production although other CaMK members also seem to contribute to this event. Furthermore, the CaMK inhibitor treatment reduced IFN-beta production in a murine endotoxemia. Our results suggest CaMKs contribute to HMGB1 release by enhancing IFN-beta production in sepsis.
Keyword
MeSH Terms
-
Benzimidazoles
Cytokines
Endotoxemia
HMGB1 Protein
Inflammation
Interferon Regulatory Factor-3
Interferon-beta
Molecular Biology
Naphthalimides
Phosphorylation
Phosphotransferases
Protein Kinases
Sepsis
Signal Transduction
Benzimidazoles
Cytokines
HMGB1 Protein
Interferon Regulatory Factor-3
Interferon-beta
Naphthalimides
Phosphotransferases
Protein Kinases
Figure
Reference
-
1. Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010. 28:367–388.
Article2. Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005. 5:331–342.
Article3. Degryse B, Bonaldi T, Scaffidi P, Müller S, Resnati M, Sanvito F, Arrigoni G, Bianchi ME. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol. 2001. 152:1197–1206.
Article4. Rouhiainen A, Kuja-Panula J, Wilkman E, Pakkanen J, Stenfors J, Tuominen RK, Lepäntalo M, Carpén O, Parkkinen J, Rauvala H. Regulation of monocyte migration by amphoterin (HMGB1). Blood. 2004. 104:1174–1182.
Article5. Sappington PL, Yang R, Yang H, Tracey KJ, Delude RL, Fink MP. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology. 2002. 123:790–802.
Article6. Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000. 192:565–570.
Article7. Youn JH, Oh YJ, Kim ES, Choi JE, Shin JS. High mobility group box 1 protein binding to lipopolysaccharide facilitates transfer of lipopolysaccharide to CD14 and enhances lipopolysaccharide-mediated TNF-alpha production in human monocytes. J Immunol. 2008. 180:5067–5074.
Article8. Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol. 2008. 180:2531–2537.
Article9. Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003. 22:5551–5560.
Article10. Kim JH, Kim SJ, Lee IS, Lee MS, Uematsu S, Akira S, Oh KI. Bacterial endotoxin induces the release of high mobility group box 1 via the IFN-beta signaling pathway. J Immunol. 2009. 182:2458–2466.
Article11. Zhang X, Wheeler D, Tang Y, Guo L, Shapiro RA, Ribar TJ, Means AR, Billiar TR, Angus DC, Rosengart MR. Calcium/calmodulin-dependent protein kinase (CaMK) IV mediates nucleocytoplasmic shuttling and release of HMGB1 during lipopolysaccharide stimulation of macrophages. J Immunol. 2008. 181:5015–5023.
Article12. Wang L, Tassiulas I, Park-Min KH, Reid AC, Gil-Henn H, Schlessinger J, Baron R, Zhang JJ, Ivashkiv LB. 'Tuning' of type I interferon-induced Jak-STAT1 signaling by calcium-dependent kinases in macrophages. Nat Immunol. 2008. 9:186–193.
Article13. Tokumitsu H, Inuzuka H, Ishikawa Y, Ikeda M, Saji I, Kobayashi R. STO-609, a specific inhibitor of the Ca(2+)/calmodulin-dependent protein kinase kinase. J Biol Chem. 2002. 277:15813–15818.
Article14. Karaghiosoff M, Steinborn R, Kovarik P, Kriegshäuser G, Baccarini M, Donabauer B, Reichart U, Kolbe T, Bogdan C, Leanderson T, Levy D, Decker T, Müller M. Central role for type I interferons and Tyk2 in lipopolysaccharide-induced endotoxin shock. Nat Immunol. 2003. 4:471–477.
Article15. Weighardt H, Kaiser-Moore S, Schlautkötter S, Rossmann-Bloeck T, Schleicher U, Bogdan C, Holzmann B. Type I IFN modulates host defense and late hyperinflammation in septic peritonitis. J Immunol. 2006. 177:5623–5630.
Article16. Liu X, Yao M, Li N, Wang C, Zheng Y, Cao X. CaMKII promotes TLR-triggered proinflammatory cytokine and type I interferon production by directly binding and activating TAK1 and IRF3 in macrophages. Blood. 2008. 112:4961–4970.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Calmodulin in the Generation of Reactive Oxygen Species and Apoptosis Induced by Tamoxifen in HepG2 Human Hepatoma Cells
- PKR as a Regulator of Inflammasome Activation
- Ras Mitogen-activated Protein Kinase Signaling and Kinase Suppressor of Ras as Therapeutic Targets for Hepatocellular Carcinoma
- A Memory Molecule, Ca2+/Calmodulin-Dependent Protein Kinase II and Redox Stress; Key Factors for Arrhythmias in a Diseased Heart
- Regulation of Protein Kinase in KCl-induced Contraction of Cat Gastric Smooth Muscle