Allergy Asthma Immunol Res.
2017 Nov;9(6):540-549. 10.4168/aair.2017.9.6.540.
Mouse Model of IL-17-Dominant Rhinitis Using Polyinosinic-Polycytidylic Acid
- Affiliations
-
- 1Department of Premedical Course, Dankook University College of Medicine, Cheonan, Korea.
- 2Department of Otorhinolaryngology, Dankook University College of Medicine, Cheonan, Korea. jihunmo@gmail.com
- 3Beckman Laser Institute Korea, Dankook University College of Medicine, Cheonan, Korea.
- KMID: 2421665
- DOI: http://doi.org/10.4168/aair.2017.9.6.540
Abstract
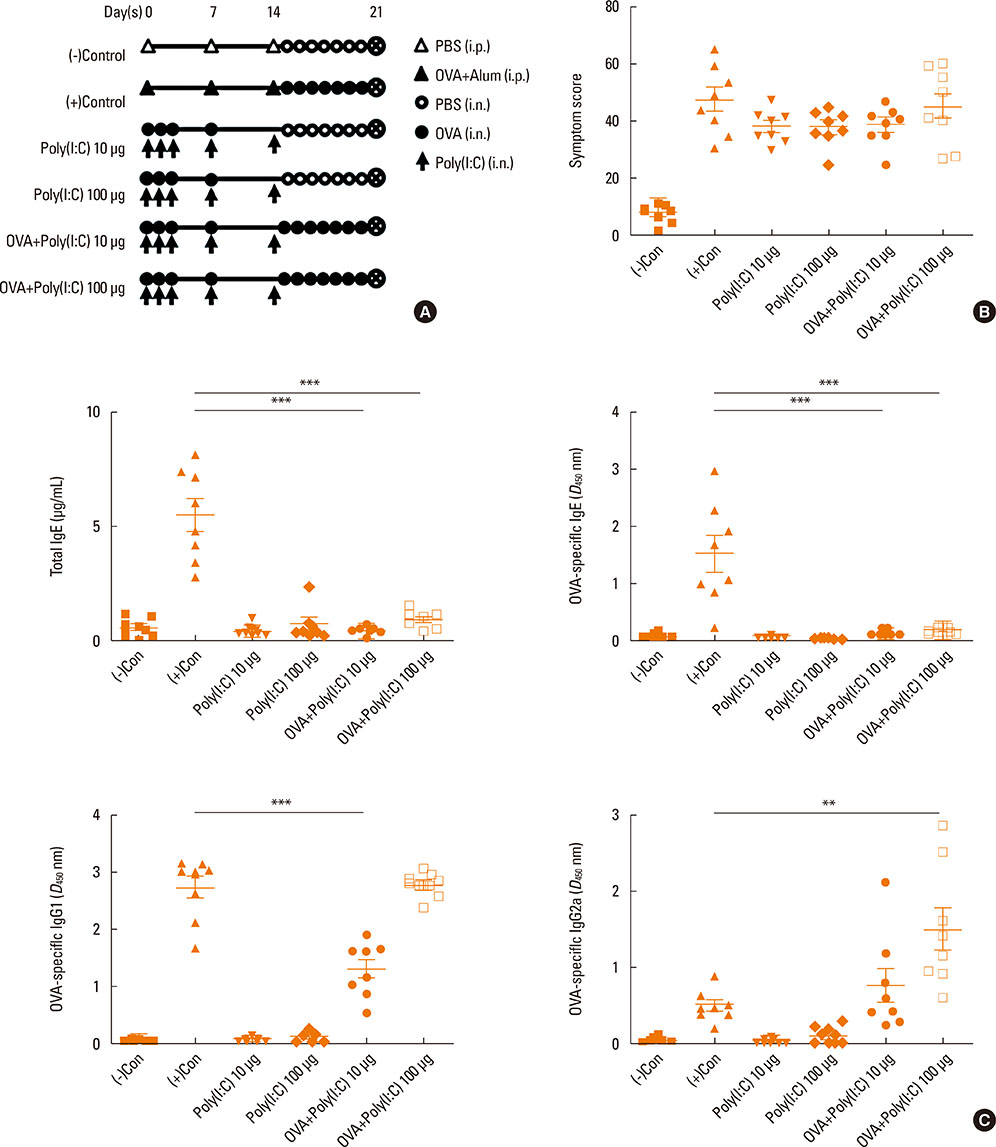
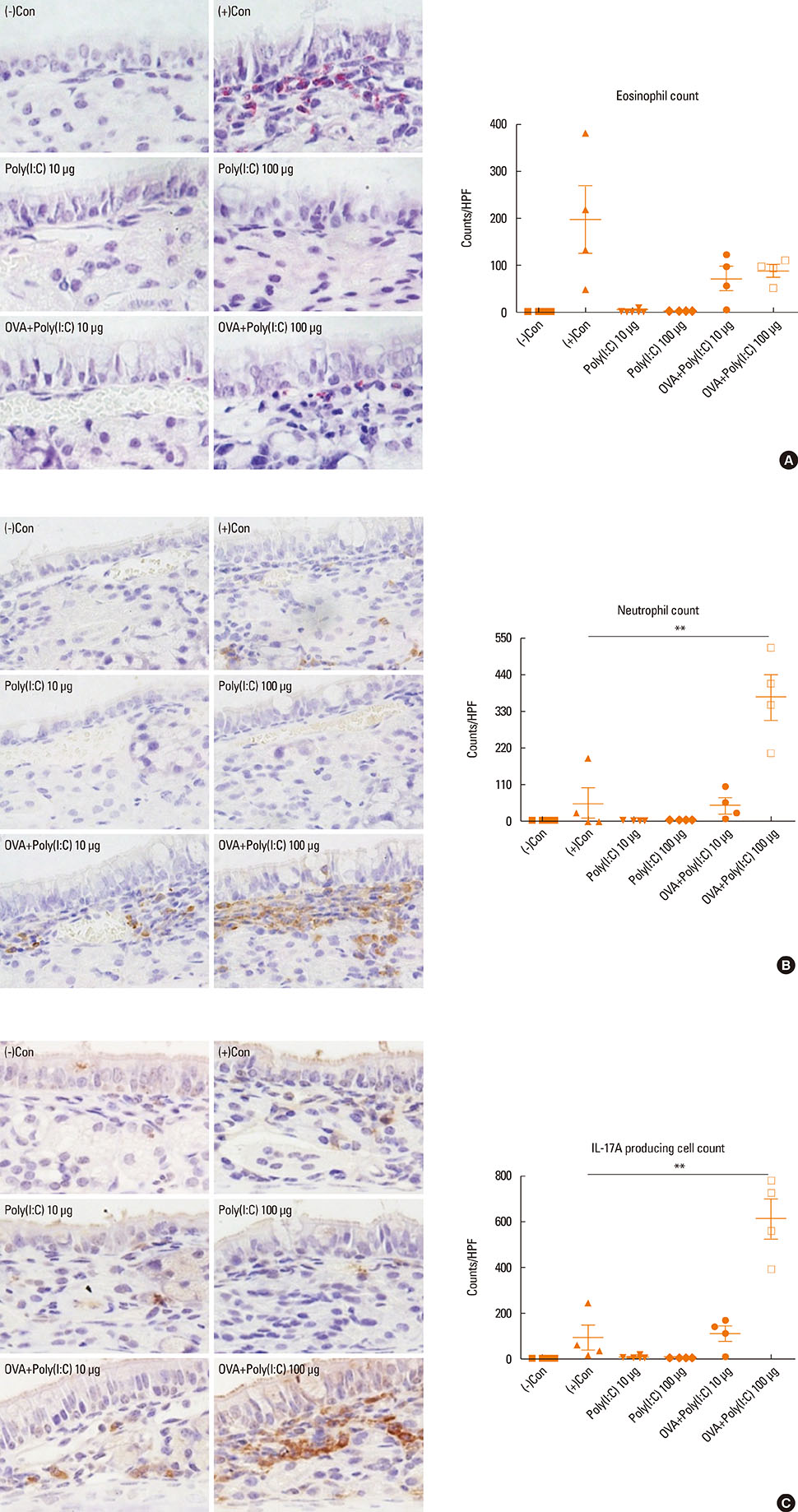
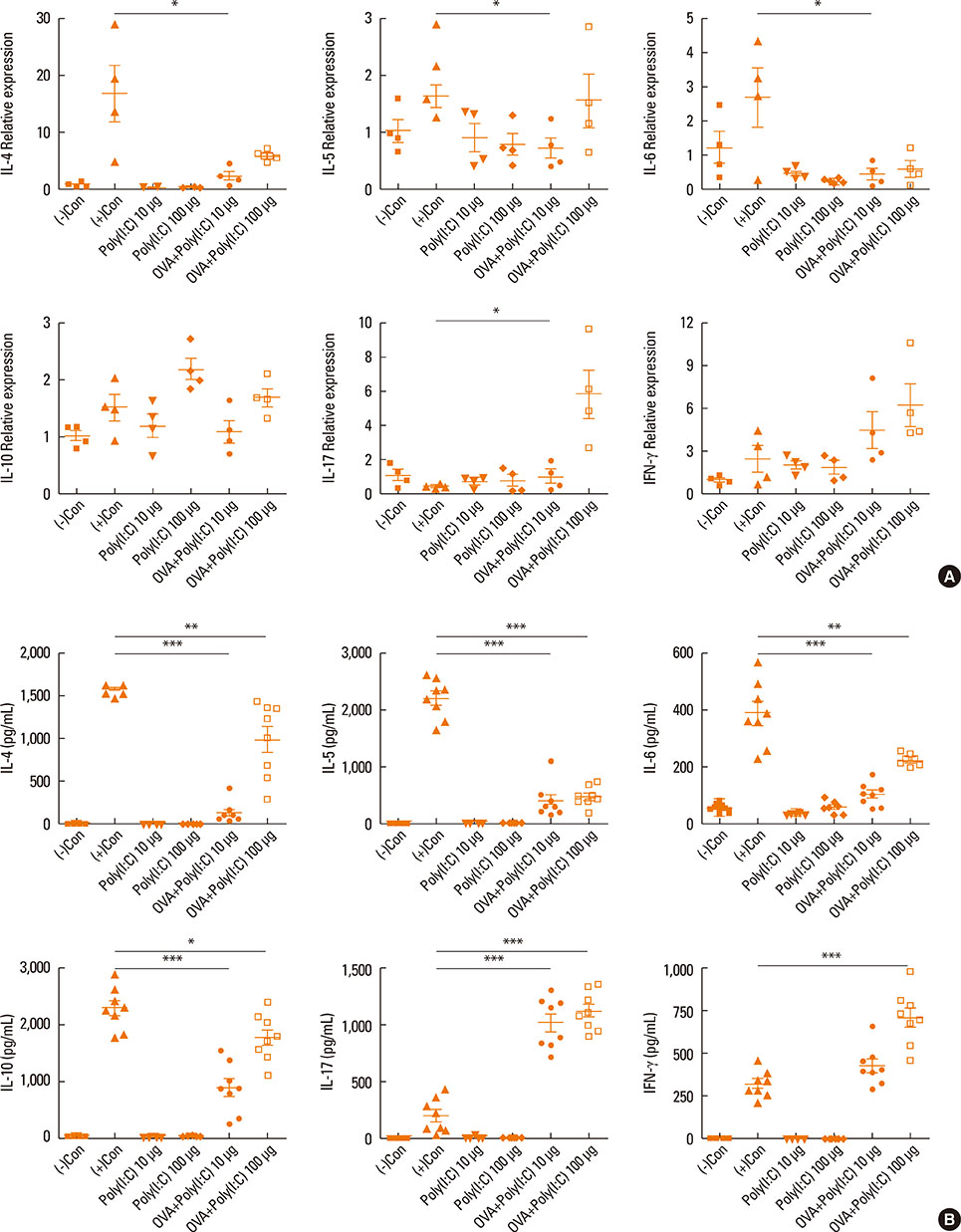
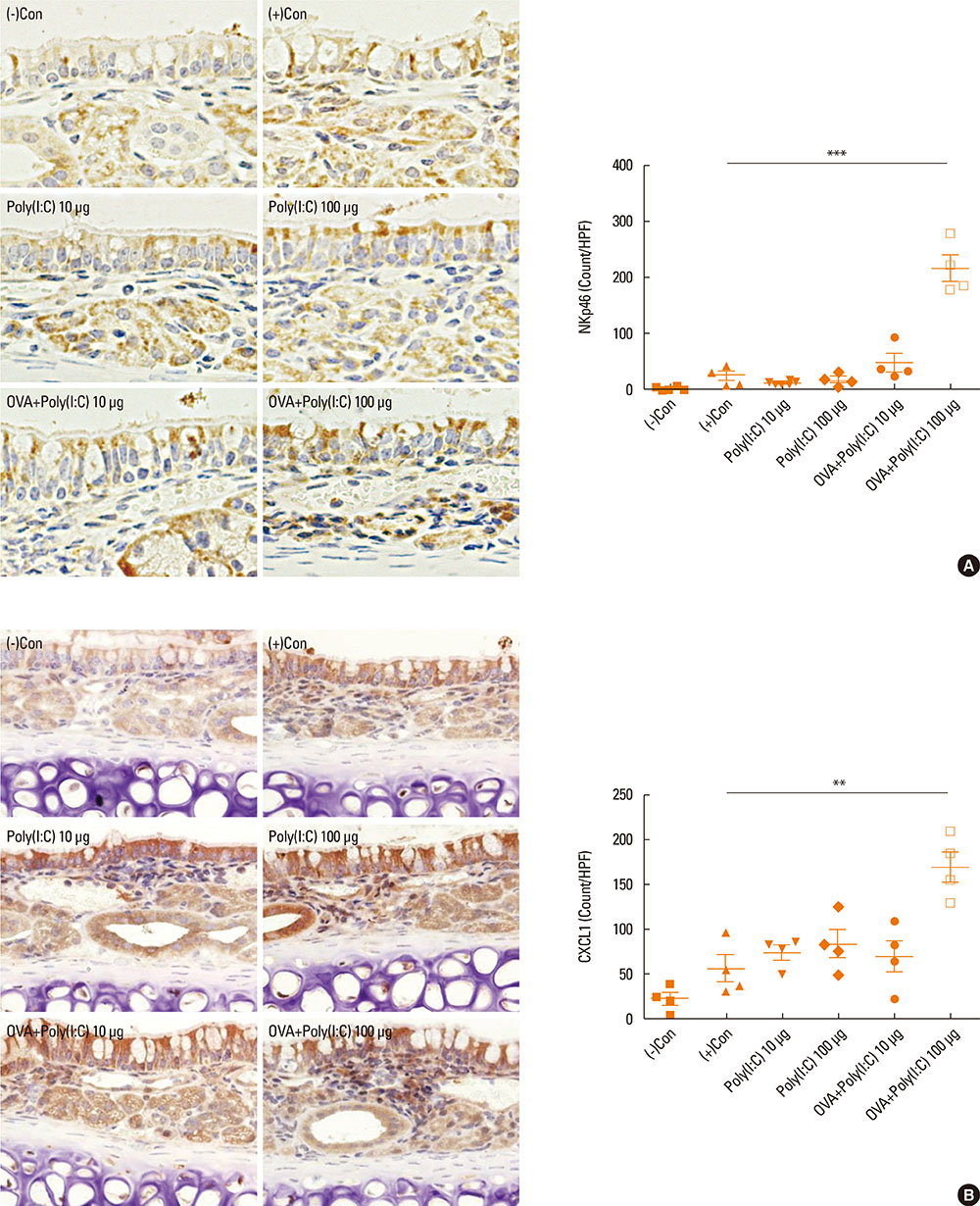
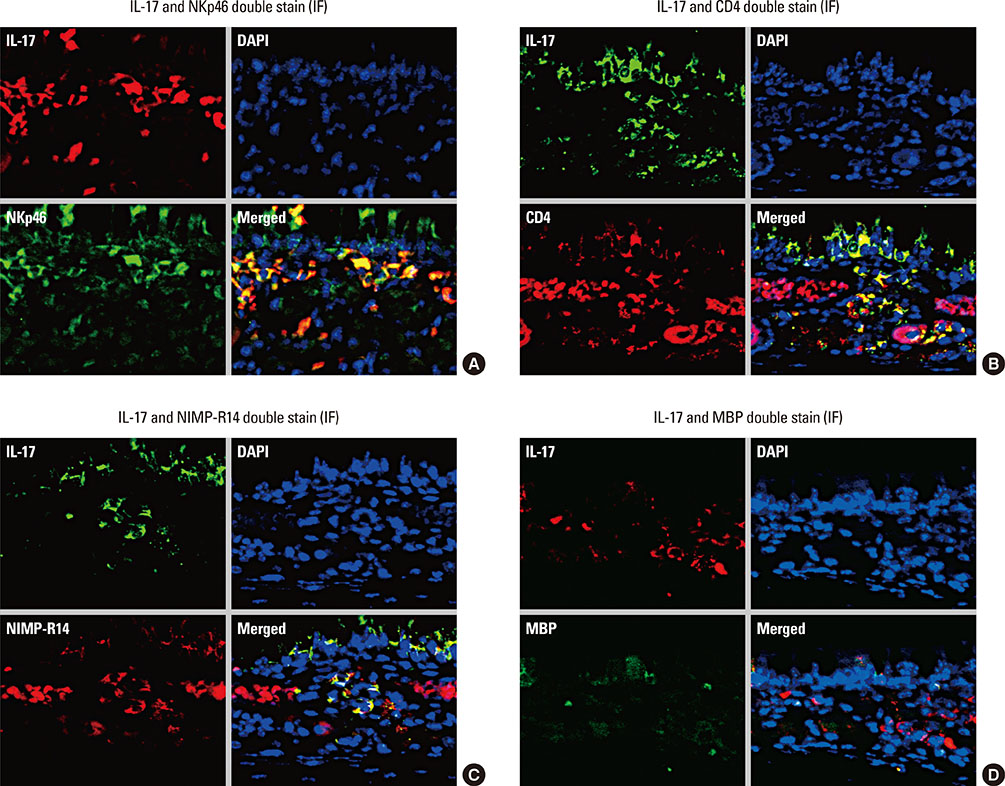
- Interleukin (IL)-17 plays an important role in rhinitis and the level thereof correlates with the severity of disease. However, no mouse model for IL-17-dominant rhinitis has yet been developed. Our objective was to establish a mouse model of IL-17-dominant rhinitis via intranasal application of polyinosinic-polycytidylic acid (abbreviated as Poly(I:C)). Mice were divided into 6 groups (n=8 for each group); 1) 1 negative control group, 2) 1 positive control group (OVA/alum model), 3) 2 Poly(I:C) groups (10 or 100 µg), and 4) 2 OVA/Poly(I:C) groups (10 or 100 µg). The positive control group was treated with the conventional OVA/alum protocol. In the Poly(I:C) and OVA/Poly(I:C) groups, phosphate-buffered saline or an OVA solution plus Poly(I:C) were administered. The OVA/Poly(I:C) groups exhibited significantly greater neutrophil infiltration and increased IL-17/interferon γ expression compared with the other groups. However, the levels of total immunoglobulin E (IgE), OVA-specific IgE, eosinophil infiltration, IL-4, IL-5, IL-6, and IL-10 were significantly lower in the OVA/Poly(I:C) groups than in mice subjected to conventional Th2-dominant OVA/alum treatment (the positive control group). IL-17 and neutrophil measurement, chemokine (C-X-C motif) ligand 1 immunohistochemistry, and confocal microscopy revealed increased numbers of IL-17-secreting cells in the nasal mucosa of the OVA/Poly(I:C) groups, which included natural killer cells, CD4 T cells, and neutrophils. In conclusion, we developed a mouse model of IL-17-dominant rhinitis using OVA together with Poly(I:C). This model will be useful in research on neutrophil- or IL-17-dominant rhinitis.
MeSH Terms
-
Animals
Chemokine CXCL1
Eosinophils
Immunoglobulin E
Immunoglobulins
Immunohistochemistry
Interleukin-10
Interleukin-17
Interleukin-4
Interleukin-5
Interleukin-6
Interleukins
Killer Cells, Natural
Mice*
Microscopy, Confocal
Nasal Mucosa
Neutrophil Infiltration
Neutrophils
Ovum
Poly I-C*
Rhinitis*
T-Lymphocytes
Chemokine CXCL1
Immunoglobulin E
Immunoglobulins
Interleukin-10
Interleukin-17
Interleukin-4
Interleukin-5
Interleukin-6
Interleukins
Poly I-C
Figure
Reference
-
1. Weaver CT, Elson CO, Fouser LA, Kolls JK. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu Rev Pathol. 2013; 8:477–512.2. Ciprandi G, De Amici M, Murdaca G, Fenoglio D, Ricciardolo F, Marseglia G, et al. Serum interleukin-17 levels are related to clinical severity in allergic rhinitis. Allergy. 2009; 64:1375–1378.3. Quan SH, Zhang YL, Han DH, Iwakura Y, Rhee CS. Contribution of interleukin 17A to the development and regulation of allergic inflammation in a murine allergic rhinitis model. Ann Allergy Asthma Immunol. 2012; 108:342–350.4. Halwani R, Al-Muhsen S, Hamid Q. T helper 17 cells in airway diseases: from laboratory bench to bedside. Chest. 2013; 143:494–501.5. Vultaggio A, Nencini F, Pratesi S, Petroni G, Romagnani S, Maggi E. Poly(I:C) promotes the production of IL-17A by murine CD1d-driven invariant NKT cells in airway inflammation. Allergy. 2012; 67:1223–1232.6. Lunding LP, Webering S, Vock C, Behrends J, Wagner C, Hölscher C, et al. Poly(inosinic-cytidylic) acid-triggered exacerbation of experimental asthma depends on IL-17A produced by NK cells. J Immunol. 2015; 194:5615–5625.7. Jeon SG, Oh SY, Park HK, Kim YS, Shim EJ, Lee HS, et al. TH2 and TH1 lung inflammation induced by airway allergen sensitization with low and high doses of double-stranded RNA. J Allergy Clin Immunol. 2007; 120:803–812.8. Samivel R, Kim DW, Son HR, Rhee YH, Kim EH, Kim JH, et al. The role of TRPV1 in the CD4+ T cell-mediated inflammatory response of allergic rhinitis. Oncotarget. 2016; 7:148–160.9. Barnes PJ. New molecular targets for the treatment of neutrophilic diseases. J Allergy Clin Immunol. 2007; 119:1055–1062.10. Saito H, Matsumoto K, Denburg AE, Crawford L, Ellis R, Inman MD, et al. Pathogenesis of murine experimental allergic rhinitis: a study of local and systemic consequences of IL-5 deficiency. J Immunol. 2002; 168:3017–3023.11. Hellings PW, Hessel EM, Van Den Oord JJ, Kasran A, Van Hecke P, Ceuppens JL. Eosinophilic rhinitis accompanies the development of lower airway inflammation and hyper-reactivity in sensitized mice exposed to aerosolized allergen. Clin Exp Allergy. 2001; 31:782–790.12. Hussain I, Randolph D, Brody SL, Song SK, Hsu A, Kahn AM, et al. Induction, distribution and modulation of upper airway allergic inflammation in mice. Clin Exp Allergy. 2001; 31:1048–1059.13. Albano GD, Di Sano C, Bonanno A, Riccobono L, Gagliardo R, Chanez P, et al. Th17 immunity in children with allergic asthma and rhinitis: a pharmacological approach. PLoS One. 2013; 8:e58892.14. Oboki K, Ohno T, Saito H, Nakae S. Th17 and allergy. Allergol Int. 2008; 57:121–134.15. Stowell NC, Seideman J, Raymond HA, Smalley KA, Lamb RJ, Egenolf DD, et al. Long-term activation of TLR3 by poly(I:C) induces inflammation and impairs lung function in mice. Respir Res. 2009; 10:43.16. Clarke DL, Davis NH, Majithiya JB, Piper SC, Lewis A, Sleeman MA, et al. Development of a mouse model mimicking key aspects of a viral asthma exacerbation. Clin Sci (Lond). 2014; 126:567–580.17. Takayama S, Tamaoka M, Takayama K, Okayasu K, Tsuchiya K, Miyazaki Y, et al. Synthetic double-stranded RNA enhances airway inflammation and remodelling in a rat model of asthma. Immunology. 2011; 134:140–150.18. Rhee SH, Im E, Riegler M, Kokkotou E, O’Brien M, Pothoulakis C. Pathophysiological role of Toll-like receptor 5 engagement by bacterial flagellin in colonic inflammation. Proc Natl Acad Sci U S A. 2005; 102:13610–13615.19. Ambrosini YM, Yang GX, Zhang W, Tsuda M, Shu S, Tsuneyama K, et al. The multi-hit hypothesis of primary biliary cirrhosis: polyinosinic-polycytidylic acid (poly I:C) and murine autoimmune cholangitis. Clin Exp Immunol. 2011; 166:110–120.20. Bullens DM, Decraene A, Seys S, Dupont LJ. IL-17A in human respiratory diseases: innate or adaptive immunity? Clinical implications. Clin Dev Immunol. 2013; 2013:840315.21. Jones CE, Chan K. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cells. Am J Respir Cell Mol Biol. 2002; 26:748–753.22. Prause O, Laan M, Lötvall J, Lindén A. Pharmacological modulation of interleukin-17-induced GCP-2-, GRO-alpha- and interleukin-8 release in human bronchial epithelial cells. Eur J Pharmacol. 2003; 462:193–198.23. Laan M, Lötvall J, Chung KF, Lindén A. IL-17-induced cytokine release in human bronchial epithelial cells in vitro: role of mitogen-activated protein (MAP) kinases. Br J Pharmacol. 2001; 133:200–206.24. Shin TS, Lee BJ, Tae YM, Kim YS, Jeon SG, Gho YS, et al. Role of inducible nitric oxide synthase on the development of virus-associated asthma exacerbation which is dependent on Th1 and Th17 cell responses. Exp Mol Med. 2010; 42:721–730.25. Gernez Y, Tirouvanziam R, Chanez P. Neutrophils in chronic inflammatory airway diseases: can we target them and how? Eur Respir J. 2010; 35:467–469.26. Tillie-Leblond I, Gosset P, Tonnel AB. Inflammatory events in severe acute asthma. Allergy. 2005; 60:23–29.27. Bafadhel M, McCormick M, Saha S, McKenna S, Shelley M, Hargadon B, et al. Profiling of sputum inflammatory mediators in asthma and chronic obstructive pulmonary disease. Respiration. 2012; 83:36–44.28. Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, Spoede W, et al. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010; 207:2479–2491.29. Song C, Luo L, Lei Z, Li B, Liang Z, Liu G, et al. IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J Immunol. 2008; 181:6117–6124.30. Liu X, Jin H, Zhang G, Lin X, Chen C, Sun J, et al. Intratumor IL-17-positive mast cells are the major source of the IL-17 that is predictive of survival in gastric cancer patients. PLoS One. 2014; 9:e106834.31. Taylor PR, Leal SM Jr, Sun Y, Pearlman E. Aspergillus and Fusarium corneal infections are regulated by Th17 cells and IL-17-producing neutrophils. J Immunol. 2014; 192:3319–3327.32. Passos ST, Silver JS, O’Hara AC, Sehy D, Stumhofer JS, Hunter CA. IL-6 promotes NK cell production of IL-17 during toxoplasmosis. J Immunol. 2010; 184:1776–1783.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Allergic Rhinitis Mouse Model
- The Effect of Interleukin-12 on the Immune Response in Allergic Rhinitis Mouse Model
- Decreased IgE antibody formation in mice treated with polyadenyic pollyuridylic acid and polyinosinic polycytidylic acid
- NaHCO 3- and NaCl-Type Hot Springs Enhance the Secretion of Inflammatory Cytokine Induced by Polyinosinic-Polycytidylic Acid in HaCaT Cells
- The Role of IL-17 in a Lipopolysaccharide-Induced Rhinitis Model