Nutr Res Pract.
2017 Feb;11(1):3-10. 10.4162/nrp.2017.11.1.3.
Anti-inflammatory activity of a sulfated polysaccharide isolated from an enzymatic digest of brown seaweed Sargassum horneri in RAW 264.7 cells
- Affiliations
-
- 1Laboratory of Marine Bioresource Technology, Department of Marine Life Science, School of Marine Biomedical Sciences, Jeju National University, 102 Jejudaehak-ro, Jeju 63243, Korea. youjinj@jejunu.ac.kr
- 2Department of Marine Bio Food Science, Chonnam National University, Yeosu 59626, Korea. gnahn@jnu.ac.kr
- 3Department of Veterinary Medicine and Veterinary Medical Research Institute, Jeju National University, Jeju 63243, Korea.
- KMID: 2395287
- DOI: http://doi.org/10.4162/nrp.2017.11.1.3
Abstract
- BACKGROUND/OBJECTIVES
Sargassum horneri is an edible brown alga that grows in the subtidal zone as an annual species along the coasts of South Korea, China, and Japan. Recently, an extreme amount of S. horneri moved into the coasts of Jeju Island from the east coast of China, which made huge economic and environmental loss to the Jeju Island. Thus, utilization of this biomass becomes a big issue with the local authorities. Therefore, the present study was performed to evaluate the anti-inflammatory potential of crude polysaccharides (CPs) extracted from S. horneri China strain in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells.
MATERIALS/METHODS
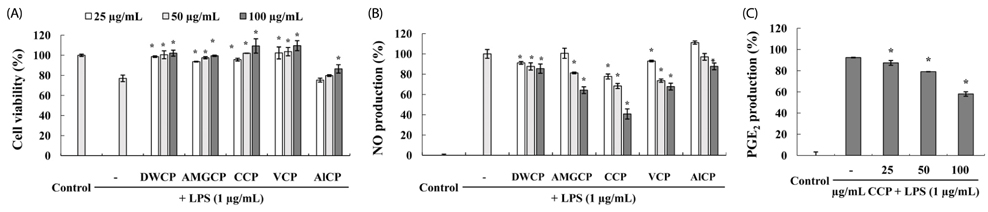
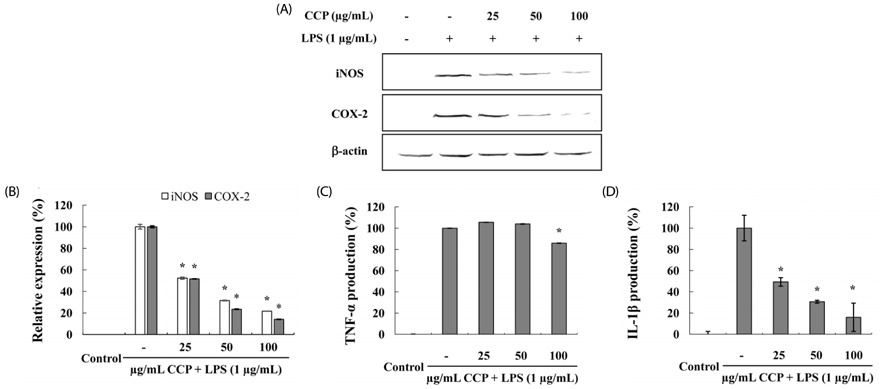
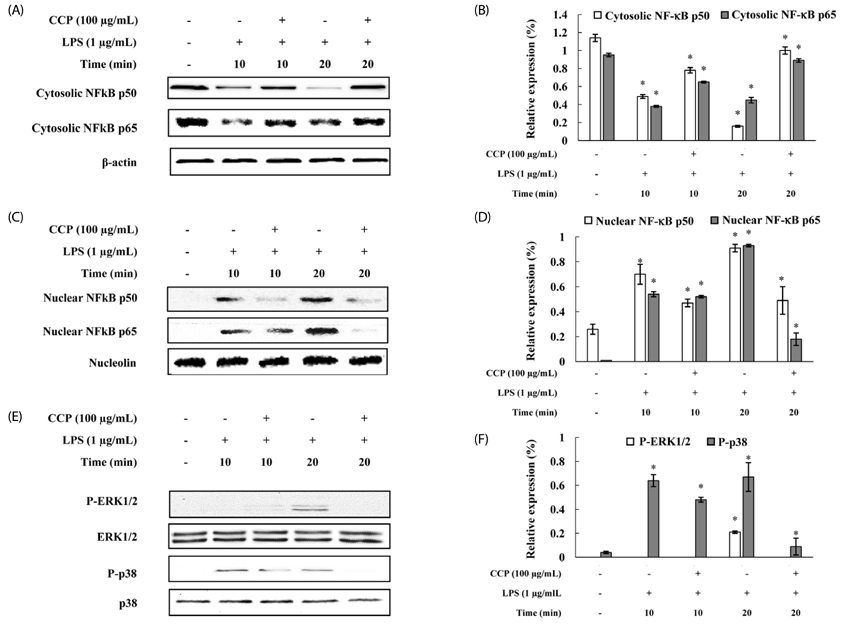
CPs were precipitated from S. horneri digests prepared by enzyme assistant extraction using four food-grade enzymes (AMG, Celluclast, Viscozyme, and Alcalase). The production levels of nitric oxide (NO) and pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α and interleukin (IL)-1β were measured by Griess assay and enzyme-linked immunosorbent assay, respectively. The levels of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), nuclear factor (NF)-κB, and mitogen-activated protein kinases (MAPKs) were measured by using western blot. The IR spectrums of the CPs were recorded using a fourier transform infrared spectroscopy (FT-IR) spectrometer.
RESULTS
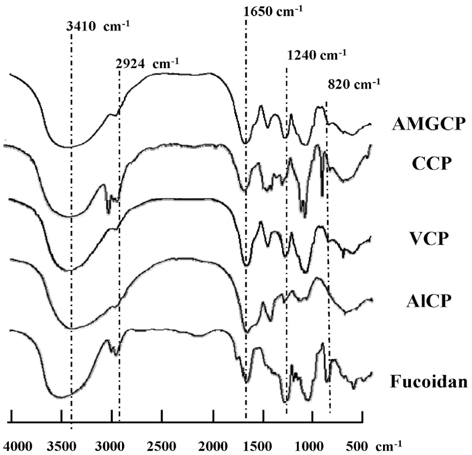
The polysaccharides from the Celluclast enzyme digest (CCP) showed the highest inhibition of NO production in LPS-stimulated RAW 264.7 cells (ICâ‚…â‚€ value: 95.7 µg/mL). Also, CCP dose-dependently down-regulated the protein expression levels of iNOS and COX-2 as well as the production of inflammatory cytokines, including TNF-α and IL-1β, compared to the only LPS-treated cells. In addition, CCP inhibited the activation of NF-κB p50 and p65 and the phosphorylation of MAPKs, including p38 and extracellular signal-regulated kinase, in LPS-stimulated RAW 264.7 cells. Furthermore, FT-IR analysis showed that the FT-IR spectrum of CCP is similar to that of commercial fucoidan.
CONCLUSIONS
Our results suggest that CCP has anti-inflammatory activities and is a potential candidate for the formulation of a functional food ingredient or/and drug to treat inflammatory diseases.
MeSH Terms
-
Biomass
Blotting, Western
China
Cyclooxygenase 2
Cytokines
Enzyme-Linked Immunosorbent Assay
Functional Food
Inflammation
Interleukins
Japan
Korea
Macrophages
Mitogen-Activated Protein Kinases
Nitric Oxide
Nitric Oxide Synthase Type II
Phosphorylation
Phosphotransferases
Polysaccharides
RAW 264.7 Cells*
Sargassum*
Seaweed*
Spectroscopy, Fourier Transform Infrared
Tumor Necrosis Factor-alpha
Cyclooxygenase 2
Cytokines
Interleukins
Mitogen-Activated Protein Kinases
Nitric Oxide
Nitric Oxide Synthase Type II
Phosphotransferases
Polysaccharides
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992; 6:3051–3064.
Article2. Knöferl MW, Diodato MD, Schwacha MG, Cioffi WG, Bland KI, Chaudry IH. Cyclooxygenase-2-mediated regulation of Kupffer cell interleukin-6 production following trauma-hemorrhage and subsequent sepsis. Shock. 2001; 16:479–483.
Article3. Louis E, Franchimont D, Piron A, Gevaert Y, Schaaf-Lafontaine N, Roland S, Mahieu P, Malaise M, De Groote D, Louis R, Belaiche J. Tumour necrosis factor (TNF) gene polymorphism influences TNF-α production in lipopolysaccharide (LPS)-stimulated whole blood cell culture in healthy humans. Clin Exp Immunol. 1998; 113:401–406.
Article4. Simmons TL, Andrianasolo E, McPhail K, Flatt P, Gerwick WH. Marine natural products as anticancer drugs. Mol Cancer Ther. 2005; 4:333–342.5. Sanjeewa KK, Kim EA, Son KT, Jeon YJ. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: a review. J Photochem Photobiol B. 2016; 162:100–105.
Article6. Wijesekara I, Pangestuti R, Kim SK. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr Polym. 2011; 84:14–21.
Article7. Wijesinghe WA, Jeon YJ. Enzyme-assistant extraction (EAE) of bioactive components: a useful approach for recovery of industrially important metabolites from seaweeds: a review. Fitoterapia. 2012; 83:6–12.
Article8. Heo SJ, Jeon YJ, Lee JH, Kim HT, Lee KW. Antioxidant effect of enzymatic hydrolyzate from a Kelp, Ecklonia cava. Algae. 2003; 18:341–347.
Article9. Pang SJ, Liu F, Shan TF, Gao SQ, Zhang ZH. Cultivation of the brown alga Sargassum horneri: sexual reproduction and seedling production in tank culture under reduced solar irradiance in ambient temperature. J Appl Phycol. 2009; 21:413–422.
Article10. Liu L, Heinrich M, Myers S, Dworjanyn SA. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in traditional Chinese medicine: a phytochemical and pharmacological review. J Ethnopharmacol. 2012; 142:591–619.
Article11. Preeprame S, Hayashi K, Lee JB, Sankawa U, Hayashi T. A novel antivirally active fucan sulfate derived from an edible brown alga, Sargassum horneri. Chem Pharm Bull (Tokyo). 2001; 49:484–485.
Article12. Shao P, Chen X, Sun P. Chemical characterization, antioxidant and antitumor activity of sulfated polysaccharide from Sargassum horneri. Carbohydr Polym. 2014; 105:260–269.
Article13. Shao P, Chen X, Sun P. Improvement of antioxidant and moisture-preserving activities of Sargassum horneri polysaccharide enzymatic hydrolyzates. Int J Biol Macromol. 2015; 74:420–427.
Article14. DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956; 28:350–356.
Article15. Chandler SF, Dodds JH. The effect of phosphate, nitrogen and sucrose on the production of phenolics and solasodine in callus cultures of solanum laciniatum. Plant Cell Rep. 1983; 2:205–208.
Article16. Saito H, Yamagata T, Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968; 243:1536–1542.
Article17. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.
Article18. Leiro J, Alvarez E, García D, Orallo F. Resveratrol modulates rat macrophage functions. Int Immunopharmacol. 2002; 2:767–774.
Article19. Park JW, Choi YJ, Suh SI, Kwon TK. Involvement of ERK and protein tyrosine phosphatase signaling pathways in EGCG-induced cyclooxygenase-2 expression in Raw 264.7 cells. Biochem Biophys Res Commun. 2001; 286:721–725.
Article20. Yang L, Zhang LM. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydr Polym. 2009; 76:349–361.
Article21. Patankar MS, Oehninger S, Barnett T, Williams RL, Clark GF. A revised structure for fucoidan may explain some of its biological activities. J Biol Chem. 1993; 268:21770–21776.
Article22. Bernardi G, Springer GF. Properties of highly purified fucan. J Biol Chem. 1962; 237:75–80.
Article23. Qiu X, Amarasekara A, Doctor V. Effect of oversulfation on the chemical and biological properties of fucoidan. Carbohydr Polym. 2006; 63:224–228.
Article24. Wijesinghe WA, Athukorala Y, Jeon YJ. Effect of anticoagulative sulfated polysaccharide purified from enzyme-assistant extract of a brown seaweed Ecklonia cava on Wistar rats. Carbohydr Polym. 2011; 86:917–921.
Article25. Gómez-Ordóñez E, Rupérez P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll. 2011; 25:1514–1520.
Article26. Chen Y, Mao W, Gao Y, Teng X, Zhu W, Chen Y, Zhao C, Li N, Wang C, Yan M, Shan J, Lin C, Guo T. Structural elucidation of an extracellular polysaccharide produced by the marine fungus Aspergillus versicolor. Carbohydr Polym. 2013; 93:478–483.
Article27. Bertolini A, Ottani A, Sandrini M. Dual acting anti-inflammatory drugs: a reappraisal. Pharmacol Res. 2001; 44:437–450.
Article28. Tilg H, Wilmer A, Vogel W, Herold M, Nölchen B, Judmaier G, Huber C. Serum levels of cytokines in chronic liver diseases. Gastroenterology. 1992; 103:264–274.
Article29. Sestini P, Armetti L, Gambaro G, Pieroni MG, Refini RM, Sala A, Vaghi A, Folco GC, Bianco S, Robuschi M. Inhaled PGE2 prevents aspirin-induced bronchoconstriction and urinary LTE4 excretion in aspirin-sensitive asthma. Am J Respir Crit Care Med. 1996; 153:572–575.
Article30. Wijesinghe WA, Jeon YJ. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: a review. Carbohydr Polym. 2012; 88:13–20.
Article31. Park HY, Han MH, Park C, Jin CY, Kim GY, Choi IW, Kim ND, Nam TJ, Kwon TK, Choi YH. Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem Toxicol. 2011; 49:1745–1752.
Article32. Wu GJ, Shiu SM, Hsieh MC, Tsai GJ. Anti-inflammatory activity of a sulfated polysaccharide from the brown alga Sargassum cristaefolium. Food Hydrocoll. 2016; 53:16–23.
Article33. Posadas I, Terencio MC, Guillén I, Ferrándiz ML, Coloma J, Payá M, Alcaraz MJ. Co-regulation between cyclo-oxygenase-2 and inducible nitric oxide synthase expression in the time-course of murine inflammation. Naunyn Schmiedebergs Arch Pharmacol. 2000; 361:98–106.
Article34. Attur MG, Patel R, Thakker G, Vyas P, Levartovsky D, Patel P, Naqvi S, Raza R, Patel K, Abramson D, Bruno G, Abramson SB, Amin AR. Differential anti-inflammatory effects of immunosuppressive drugs: cyclosporin, rapamycin and FK-506 on inducible nitric oxide synthase, nitric oxide, cyclooxygenase-2 and PGE2 production. Inflamm Res. 2000; 49:20–26.
Article35. Heo SJ, Yoon WJ, Kim KN, Ahn GN, Kang SM, Kang DH, Affan A, Oh C, Jung WK, Jeon YJ. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem Toxicol. 2010; 48:2045–2051.
Article36. Tews DS, Goebel HH. Cytokine expression profile in idiopathic inflammatory myopathies. J Neuropathol Exp Neurol. 1996; 55:342–347.
Article37. Jung WK, Ahn YW, Lee SH, Choi YH, Kim SK, Yea SS, Choi I, Park SG, Seo SK, Lee SW, Choi IW. Ecklonia cava ethanolic extracts inhibit lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression in BV2 microglia via the MAP kinase and NF-κB pathways. Food Chem Toxicol. 2009; 47:410–417.
Article38. Kang JS, Yoon YD, Lee KH, Park SK, Kim HM. Costunolide inhibits interleukin-1β expression by down-regulation of AP-1 and MAPK activity in LPS-stimulated RAW 264.7 cells. Biochem Biophys Res Commun. 2004; 313:171–177.
Article39. Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-κB and IκB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005; 30:43–52.
Article40. Xiao YQ, Malcolm K, Worthen GS, Gardai S, Schiemann WP, Fadok VA, Bratton DL, Henson PM. Cross-talk between ERK and p38 MAPK mediates selective suppression of pro-inflammatory cytokines by transforming growth factor-β. J Biol Chem. 2002; 277:14884–14893.
Article41. Aga M, Watters JJ, Pfeiffer ZA, Wiepz GJ, Sommer JA, Bertics PJ. Evidence for nucleotide receptor modulation of cross talk between MAP kinase and NF-κB signaling pathways in murine RAW 264.7 macrophages. Am J Physiol Cell Physiol. 2004; 286:C923–C930.
Article42. DeFranco AL, Hambleton J, McMahon M, Weinstein SL. Examination of the role of MAP kinase in the response of macrophages to lipopolysaccharide. Prog Clin Biol Res. 1995; 392:407–420.43. Mahtani KR, Brook M, Dean JL, Sully G, Saklatvala J, Clark AR. Mitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor alpha mRNA stability. Mol Cell Biol. 2001; 21:6461–6469.
Article44. Koistinaho M, Koistinaho J. Role of p38 and p44/42 mitogen-activated protein kinases in microglia. Glia. 2002; 40:175–183.
Article45. Tegeder I, Pfeilschifter J, Geisslinger G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J. 2001; 15:2057–2072.
Article46. Yang JW, Yoon SY, Oh SJ, Kim SK, Kang KW. Bifunctional effects of fucoidan on the expression of inducible nitric oxide synthase. Biochem Biophys Res Commun. 2006; 346:345–350.
Article47. Ale MT, Mikkelsen JD, Meyer AS. Important determinants for fucoidan bioactivity: a critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar Drugs. 2011; 9:2106–2130.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunomodulating activity of Sargassum horneri extracts in RAW264.7 macrophages
- Radio-protective effect of sulfated polysaccharide purified from Ecklonia cava against small intestinal stem cells of gamma-ray irradiated mice
- Polysaccharide from Hizikia Fusiformis Enhances the Immunomodulatory Activity of Macrophages
- Inhibitory effects of Sargassum horneri extract against endoplasmic reticulum stress in HepG2 cells
- Bioassay-Guided Isolation and Identification of Compounds from Arecae Pericarpium with Anti-inflammatory, Anti-oxidative, and Melanogenesis Inhibition Activities