Nutr Res Pract.
2017 Oct;11(5):357-364. 10.4162/nrp.2017.11.5.357.
Black soybean anthocyanins attenuate inflammatory responses by suppressing reactive oxygen species production and mitogen activated protein kinases signaling in lipopolysaccharide-stimulated macrophages
- Affiliations
-
- 1Department of Food Science and Nutrition, The Catholic University of Korea, 43 Jibong-ro, Wonmi-gu, Bucheon-si, Gyeonngi 14662, Korea. hkyeong@catholic.ac.kr
- 2Department of Food and Nutrition, Seoul National University, Seoul 08826, Korea.
- 3National Institute of Crop Science, Rural Development Administration (RDA), Wanju-gun, Jeonbuk 54875, Korea.
- KMID: 2390127
- DOI: http://doi.org/10.4162/nrp.2017.11.5.357
Abstract
- BACKGROUND/OBJECTIVES
Oxidative stress is closely related with inflammation and development of many diseases. Black soybean seed coat contains high amount of anthocyanins, which are well-known for free radical scavenging activities. This study investigated inflammatory response and action mechanism of black soybean anthocyanins with regard to antioxidant activity in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells.
MATERIALS/METHODS
RAW 264.7 cells were treated with anthocyanins extracted from black soybean seed coats in a concentration range of 12.5 to 100 µg/mL. The production of reactive oxygen species (ROS), secretion of pro-inflammatory mediators and cytokines, and the signaling in the mitogen activated protein kinases (MAPKs) pathway were examined.
RESULTS
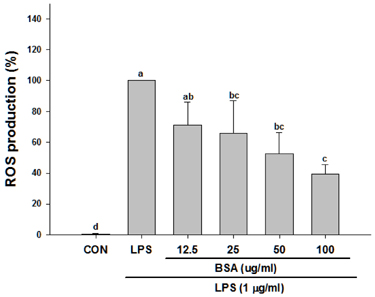
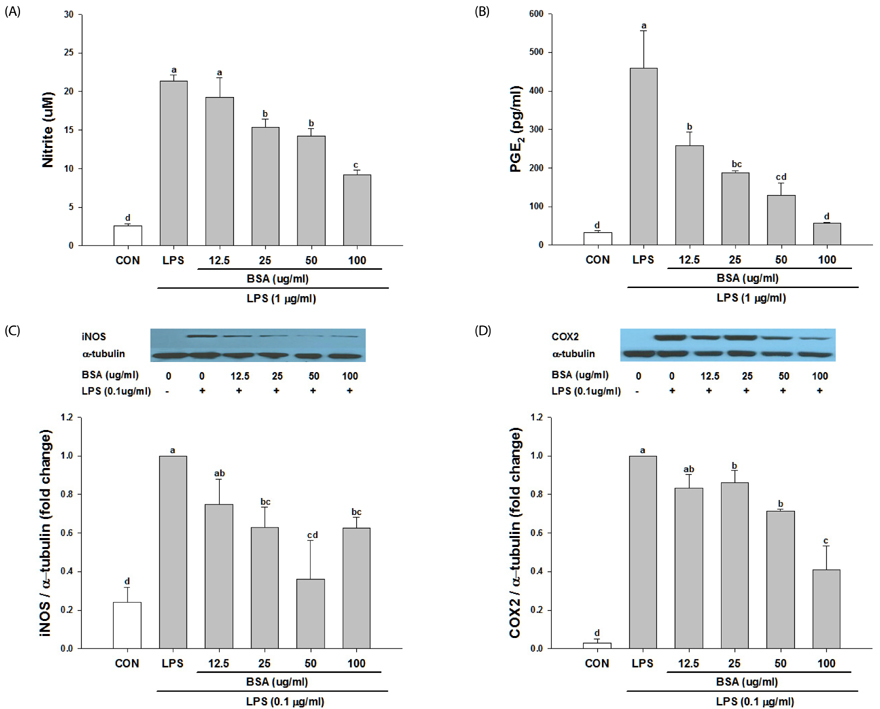
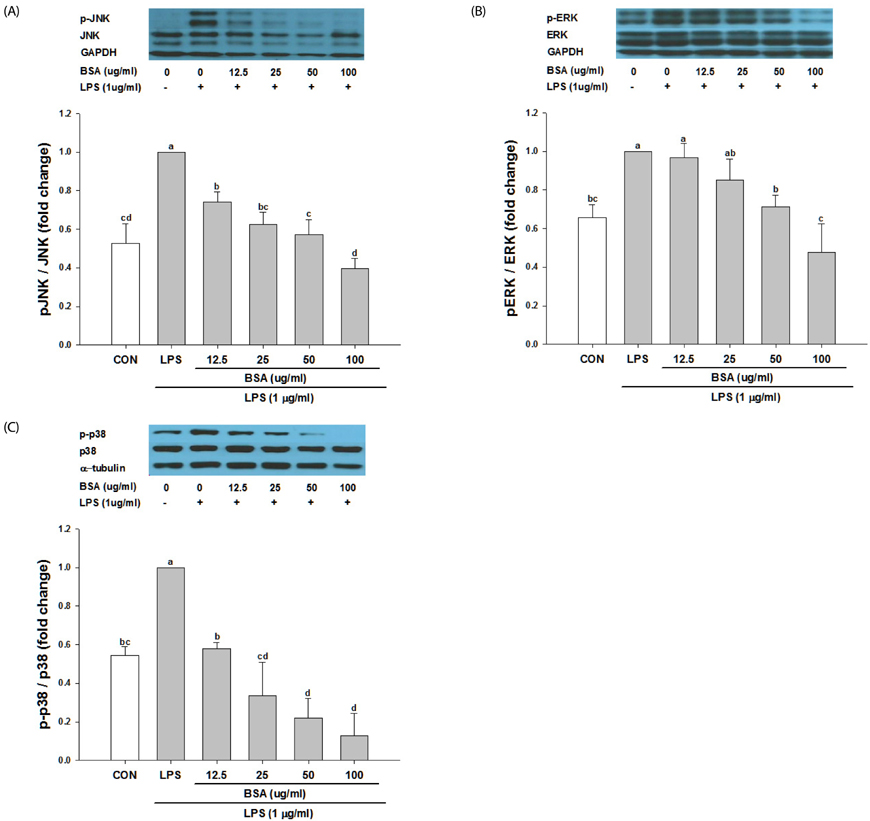
Black soybean anthocyanins significantly decreased LPS-stimulated production of ROS, inflammatory mediators such as nitric oxide (NO) and prostaglandin E₂, and pro-inflammatory cytokines, including tumor necrosis factor α and interleukin-6, in a dose-dependent manner without cytotoxicity (P < 0.001). Black soybean anthocyanins downregulated the expression of inducible NO synthase and cyclooxygenase-2 in LPS-stimulated RAW 264.7 cells (P < 0.001). Moreover, black soybean anthocyanins inhibited LPS-induced phosphorylation of MAPKs, including extracellular signal-regulated kinase, c-Jun N-terminal kinase, and p38 (P < 0.001).
CONCLUSION
These results suggest that black soybean anthocyanins exert anti-inflammatory activity by inhibiting ROS generation and subsequent MAPKs signaling, thereby inhibiting inflammatory responses.
Keyword
MeSH Terms
-
Anthocyanins*
Anti-Inflammatory Agents
Cyclooxygenase 2
Cytokines
Inflammation
Interleukin-6
JNK Mitogen-Activated Protein Kinases
Macrophages*
Mitogen-Activated Protein Kinases*
Nitric Oxide
Nitric Oxide Synthase
Oxidative Stress
Phosphorylation
Phosphotransferases
RAW 264.7 Cells
Reactive Oxygen Species*
Soybeans*
Tumor Necrosis Factor-alpha
Anthocyanins
Anti-Inflammatory Agents
Cyclooxygenase 2
Cytokines
Interleukin-6
JNK Mitogen-Activated Protein Kinases
Mitogen-Activated Protein Kinases
Nitric Oxide
Nitric Oxide Synthase
Phosphotransferases
Reactive Oxygen Species
Tumor Necrosis Factor-alpha
Figure
Cited by 1 articles
-
L -Methionine inhibits 4-hydroxy-2-nonenal accumulation and suppresses inflammation in growing rats
Zhengxuan Wang, Mingcai Liang, Hui Li, Bingxiao Liu, Lin Yang
Nutr Res Pract. 2022;16(6):729-744. doi: 10.4162/nrp.2022.16.6.729.
Reference
-
1. Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J Agric Food Chem. 2006; 54:4069–4075.
Article2. Choung MG, Baek IY, Kang ST, Han WY, Shin DC, Moon HP, Kang KH. Isolation and determination of anthocyanins in seed coats of black soybean (Glycine max (L.) Merr.). J Agric Food Chem. 2001; 49:5848–5851.
Article3. Zhang RF, Zhang FX, Zhang MW, Wei ZC, Yang CY, Zhang Y, Tang XJ, Deng YY, Chi JW. Phenolic composition and antioxidant activity in seed coats of 60 Chinese black soybean (Glycine max L. Merr.) varieties. J Agric Food Chem. 2011; 59:5935–5944.
Article4. Kim HJ, Xu L, Chang KC, Shin SC, Chung JI, Kang D, Kim SH, Hur JA, Choi TH, Kim S, Choi J. Anti-inflammatory effects of anthocyanins from black soybean seed coat on the keratinocytes and ischemiareperfusion injury in rat skin flaps. Microsurgery. 2012; 32:563–570.
Article5. Kim JM, Kim KM, Park EH, Seo JH, Song JY, Shin SC, Kang HL, Lee WK, Cho MJ, Rhee KH, Youn HS, Baik SC. Anthocyanins from black soybean inhibit Helicobacter pylori-induced inflammation in human gastric epithelial AGS cells. Microbiol Immunol. 2013; 57:366–373.
Article6. Sohn DW, Bae WJ, Kim HS, Kim SW, Kim SW. The anti-inflammatory and antifibrosis effects of anthocyanin extracted from black soybean on a Peyronie disease rat model. Urology. 2014; 84:1112–1116.
Article7. Mok JW, Chang DJ, Joo CK. Antiapoptotic effects of anthocyanin from the seed coat of black soybean against oxidative damage of human lens epithelial cell induced by H2O2. Curr Eye Res. 2014; 39:1090–1098.
Article8. Drake IM, Mapstone NP, Schorah CJ, White KL, Chalmers DM, Dixon MF, Axon AT. Reactive oxygen species activity and lipid peroxidation in Helicobacter pylori associated gastritis: relation to gastric mucosal ascorbic acid concentrations and effect of H pylori eradication. Gut. 1998; 42:768–771.
Article9. Harijith A, Ebenezer DL, Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front Physiol. 2014; 5:352.
Article10. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010; 49:1603–1616.
Article11. Lee SH, Lee SY, Son DJ, Lee H, Yoo HS, Song S, Oh KW, Han DC, Kwon BM, Hong JT. Inhibitory effect of 2‘-hydroxycinnamaldehyde on nitric oxide production through inhibition of NF-κB activation in RAW 264.7 cells. Biochem Pharmacol. 2005; 69:791–799.
Article12. Suh SJ, Chung TW, Son MJ, Kim SH, Moon TC, Son KH, Kim HP, Chang HW, Kim CH. The naturally occurring biflavonoid, ochnaflavone, inhibits LPS-induced iNOS expression, which is mediated by ERK1/2 via NF-kappaB regulation, in RAW264.7 cells. Arch Biochem Biophys. 2006; 447:136–146.
Article13. Wang H, Cao G, Prior RL. Oxygen radical absorbing capacity of anthocyanins. J Agric Food Chem. 1997; 45:304–309.
Article14. Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005; 4:281–286.
Article15. Lee JH, Kang NS, Shin SO, Shin SH, Lim SG, Suh DY, Baek IY, Park KY, Ha TJ. Characterisation of anthocyanins in the black soybean (Glycine max L.) by HPLC-DAD-ESI/MS analysis. Food Chem. 2009; 112:226–231.
Article16. Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983; 130:1910–1917.17. Kim MH, Kim JN, Han SN, Kim HK. Ursolic acid isolated from guava leaves inhibits inflammatory mediators and reactive oxygen species in LPS-stimulated macrophages. Immunopharmacol Immunotoxicol. 2015; 37:228–235.
Article18. Hashimoto N, Noda T, Kim SJ, Yamauchi H, Takigawa S, Matsuura-Endo C, Suzuki T, Han KH, Fukushima M. Colored potato extracts induce superoxide dismutase-2 mRNA via ERK1/2 pathway in HepG2 cells. Plant Foods Hum Nutr. 2010; 65:266–270.
Article19. Shih PH, Yeh CT, Yen GC. Anthocyanins induce the activation of phase II enzymes through the antioxidant response element pathway against oxidative stress-induced apoptosis. J Agric Food Chem. 2007; 55:9427–9435.
Article20. Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003; 54:469–487.21. Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012; 188:21–28.
Article22. Liu ZG. Molecular mechanism of TNF signaling and beyond. Cell Res. 2005; 15:24–27.
Article23. Singh PP, Goyal A. Interleukin-6: a potent biomarker of mycobacterial infection. Springerplus. 2013; 2:686.
Article24. Jeong JW, Lee WS, Shin SC, Kim GY, Choi BT, Choi YH. Anthocyanins downregulate lipopolysaccharide-induced inflammatory responses in BV2 microglial cells by suppressing the NF-κB and Akt/MAPKs signaling pathways. Int J Mol Sci. 2013; 14:1502–1515.
Article25. Min SW, Ryu SN, Kim DH. Anti-inflammatory effects of black rice, cyanidin-3-O-beta-D-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int Immunopharmacol. 2010; 10:959–966.
Article26. Luss H, DiSilvio M, Litton AL, Molina y Vedia L, Nussler AK, Billiar TR. Inhibition of nitric oxide synthesis enhances the expression of inducible nitric oxide synthase mRNA and protein in a model of chronic liver inflammation. Biochem Biophys Res Commun. 1994; 204:635–640.
Article27. Marcus JS, Karackattu SL, Fleegal MA, Sumners C. Cytokine-stimulated inducible nitric oxide synthase expression in astroglia: role of Erk mitogen-activated protein kinase and NF-κB. Glia. 2003; 41:152–160.
Article28. Schrader LI, Kinzenbaw DA, Johnson AW, Faraci FM, Didion SP. IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscler Thromb Vasc Biol. 2007; 27:2576–2581.
Article29. Racz B, Hanto K, Tapodi A, Solti I, Kalman N, Jakus P, Kovacs K, Debreceni B, Gallyas F Jr, Sumegi B. Regulation of MKP-1 expression and MAPK activation by PARP-1 in oxidative stress: a new mechanism for the cytoplasmic effect of PARP-1 activation. Free Radic Biol Med. 2010; 49:1978–1988.
Article30. Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, Koyasu S, Matsumoto K, Takeda K, Ichijo H. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol. 2005; 6:587–592.
Article31. Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002; 298:1911–1912.
Article32. Tsoyi K, Park HB, Kim YM, Chung JI, Shin SC, Lee WS, Seo HG, Lee JH, Chang KC, Kim HJ. Anthocyanins from black soybean seed coats inhibit UVB-induced inflammatory cylooxygenase-2 gene expression and PGE2 production through regulation of the nuclear factor-kappaB and phosphatidylinositol 3-kinase/Akt pathway. J Agric Food Chem. 2008; 56:8969–8974.
Article33. Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005; 120:649–661.
Article34. Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J Exp Med. 2011; 208:519–533.
Article35. Huo Y, Rangarajan P, Ling EA, Dheen ST. Dexamethasone inhibits the Nox-dependent ROS production via suppression of MKP-1-dependent MAPK pathways in activated microglia. BMC Neurosci. 2011; 12:49.
Article36. Emre Y, Hurtaud C, Nübel T, Criscuolo F, Ricquier D, Cassard-Doulcier AM. Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages. Biochem J. 2007; 402:271–278.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- p38 Mitogen-Activated Protein Kinase and Extracellular Signal-Regulated Kinase Regulate Nitric Oxide Production and Inflammatory Cytokine Expression in Raw Cells
- Mycobacterium tuberculosis Induces the Production of Tumor Necrosis Factor-alpha, Interleukin-6, and CXCL8 in Pulmonary Epithelial Cells Through Reactive Oxygen Species-dependent Mitogen-activated Protein Kinase Activation
- Molecular Mechanism of Reactive Oxygen Species-dependent ASK1 Activation in Innate Immunity
- Intracellular Signaling Pathways that Regulate Macrophage Chemokine Expression in Response to Mycobacterium abscessus
- YJI-7 Suppresses ROS Production and Expression of Inflammatory Mediators via Modulation of p38MAPK and JNK Signaling in RAW 264.7 Macrophages