Ann Dermatol.
2017 Oct;29(5):548-558. 10.5021/ad.2017.29.5.548.
Effects of VitabridC¹² on Skin Inflammation
- Affiliations
-
- 1Department of Dermatology, College of Medicine, The Catholic University of Korea, Seoul, Korea. tykimder@catholic.ac.kr
- KMID: 2388905
- DOI: http://doi.org/10.5021/ad.2017.29.5.548
Abstract
- BACKGROUND
VitabridC¹² is newly developed and composed of vitamin C and Vitabrid (lamellar, hydrated zinc oxide).
OBJECTIVE
In this study, we aimed to investigate the effects of VitabridC¹² on psoriasis and atopic dermatitis.
METHODS
Mice with imiquimod-induced psoriasis or Dermatophagoides farinae-induced atopic dermatitis were applied with VitabridC¹². The effects of VitabridC¹² were evaluated by clinical features, histology, and immunologic features by examining cytokines and chemokines.
RESULTS
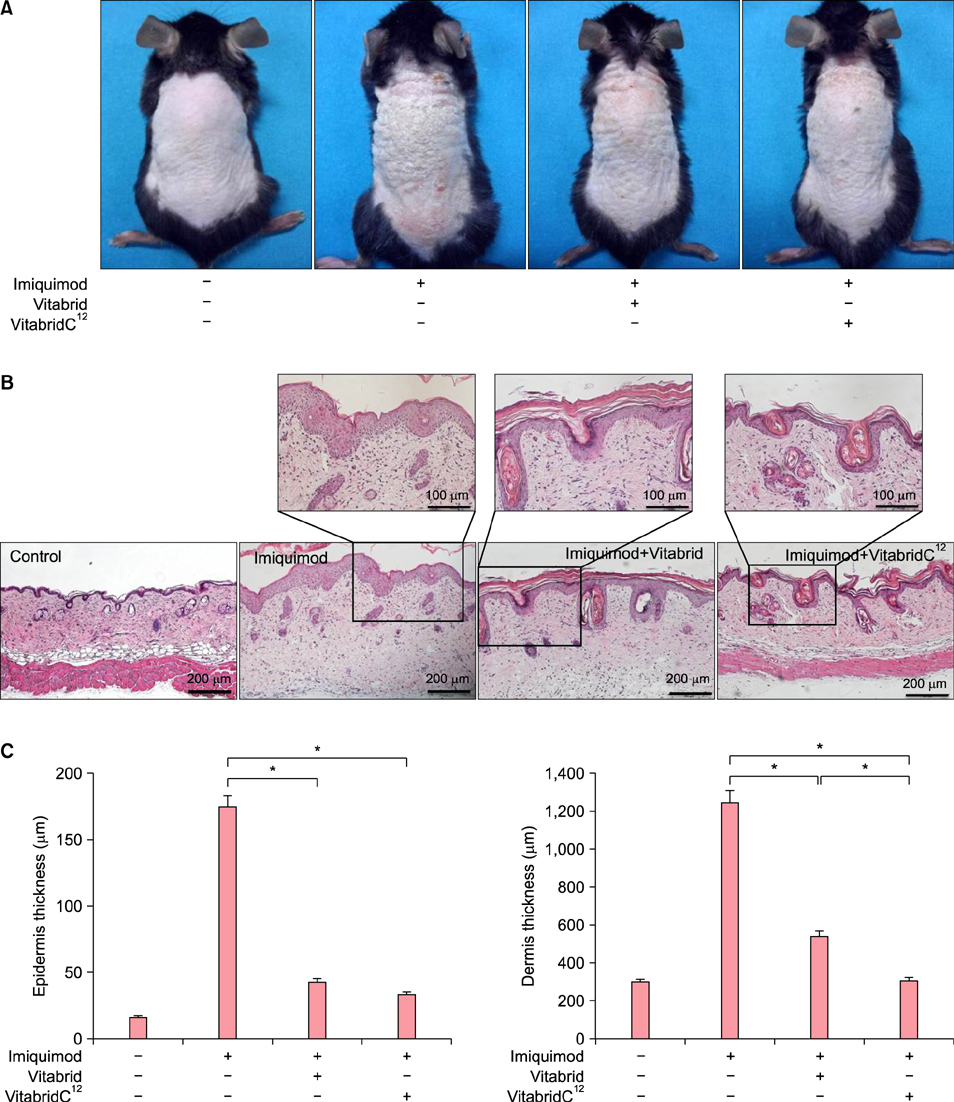
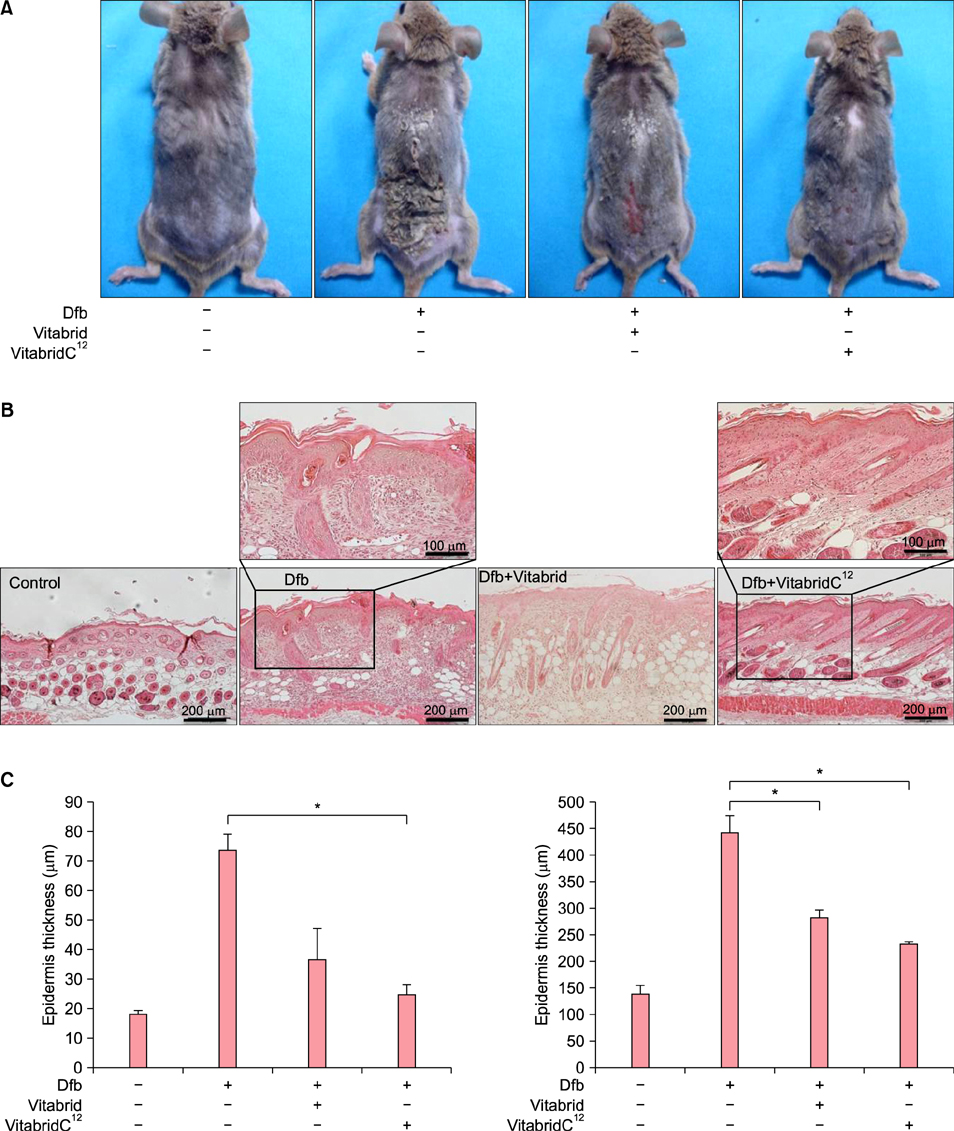
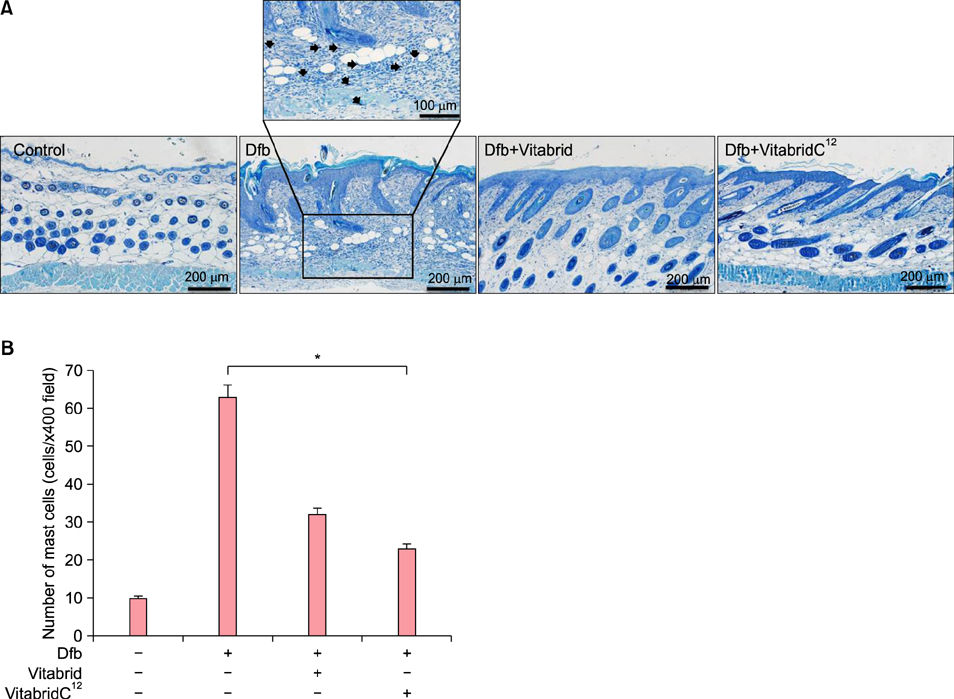
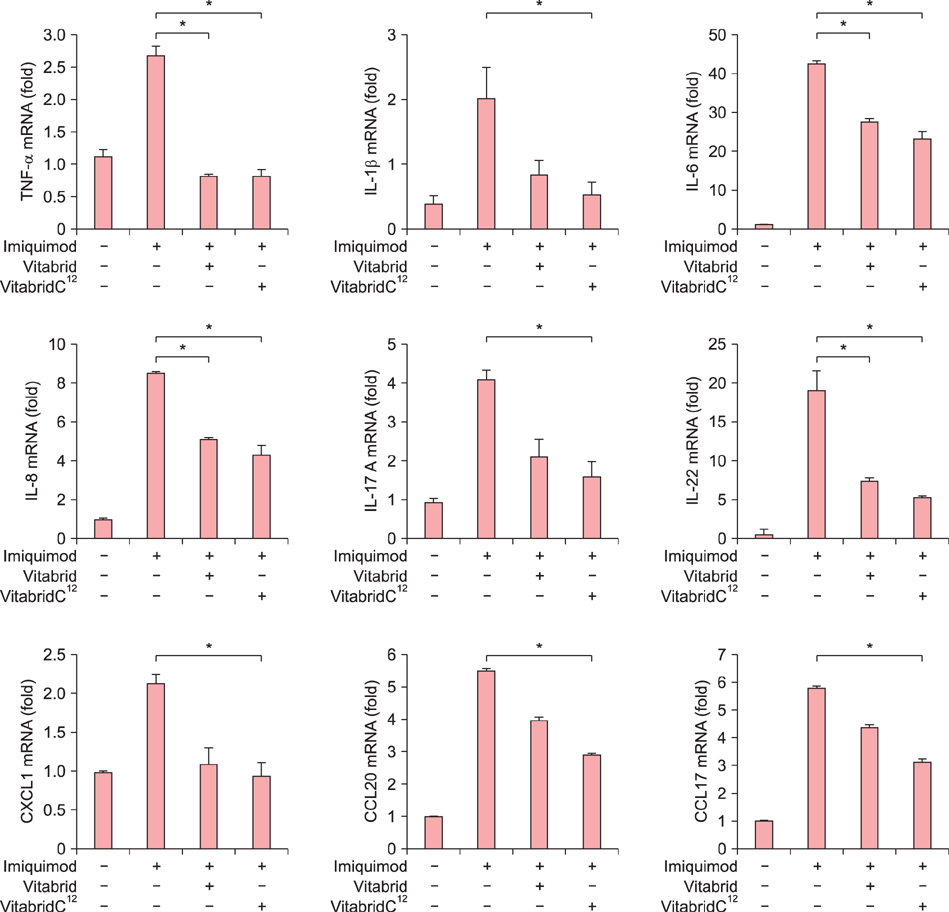
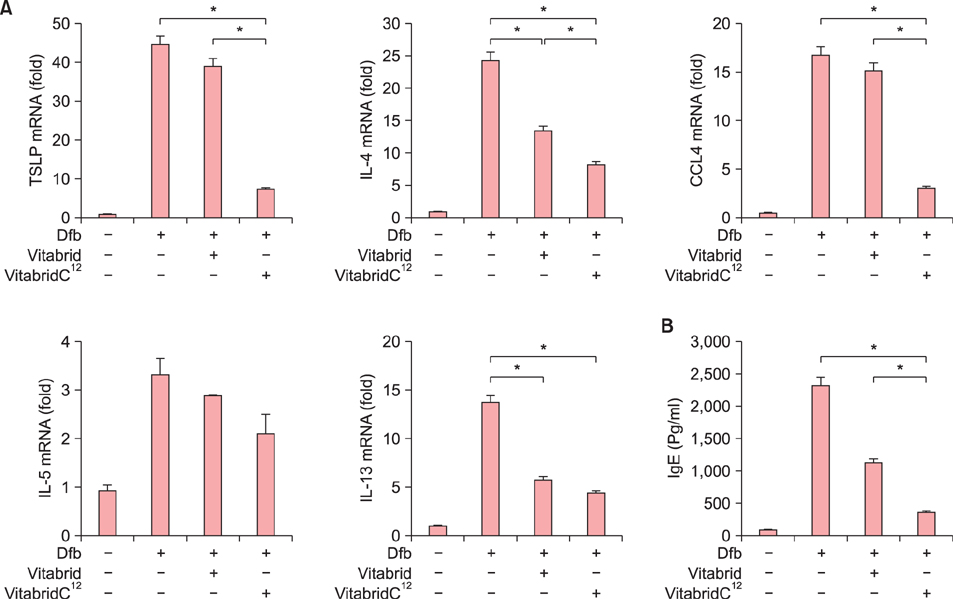
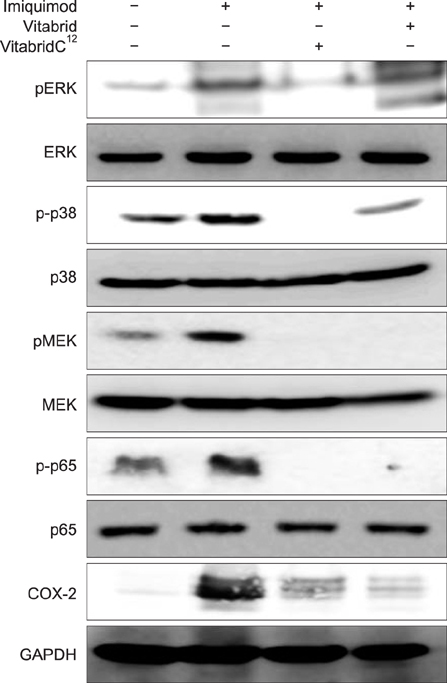
In psoriasis model, VitabridC¹² decreased epidermal thickness and reduced inflammatory cell infiltration. In atopic dermatitis model, VitabridC¹² decreased dermal infiltration of inflammatory cells, epidermal hyperplasia, and hyperkeratosis. VitabridC¹² reduced the expression levels of proinflammatory mediators such as interleukin (IL)-1β, IL-6, IL-8, IL-17A, IL-22, tumor necrosis factor-α, CXCL1, CCL17, and CCL20 as well as COX-2 in imiquimod-induced psoriatic skin lesions. Likewise, VitabridC¹² reduced the expression levels of IL-4, IL-5, IL-13, thymic stromal lymphopoietin, and CCL4 in D. farinae-induced skin lesions, and decreased the serum immunoglobulin E level in the atopic dermatitis mouse model. Particularly, the VitabridC¹²-treated mice showed downregulated expressions of mitogen-activated protein kinase (MAPK), including extracellular signal-regulated kinase (ERK), p38, and MAPK/ERK kinase, as well as inhibited phosphorylation of nuclear factor-κB p65.
CONCLUSION
Taken together, these findings indicate that VitabridC¹² exhibits anti-inflammatory activities and is a promising candidate as a treatment option for psoriasis or atopic dermatitis.
Keyword
MeSH Terms
-
Animals
Ascorbic Acid
Chemokines
Cytokines
Dermatitis, Atopic
Hyperplasia
Immunoglobulin E
Immunoglobulins
Inflammation*
Interleukin-13
Interleukin-17
Interleukin-4
Interleukin-5
Interleukin-6
Interleukin-8
Interleukins
Mice
Necrosis
Phosphorylation
Phosphotransferases
Protein Kinases
Psoriasis
Pyroglyphidae
Skin*
Zinc
Ascorbic Acid
Chemokines
Cytokines
Immunoglobulin E
Immunoglobulins
Interleukin-13
Interleukin-17
Interleukin-4
Interleukin-5
Interleukin-6
Interleukin-8
Interleukins
Phosphotransferases
Protein Kinases
Zinc
Figure
Reference
-
1. Lee JH, Jung KE, Lee YB, Kim JE, Kim HS, Lee KH, et al. Use of emollients in atopic dermatitis: a questionnaire survey study. Ann Dermatol. 2014; 26:528–531.
Article2. Koo J. Population-based epidemiologic study of psoriasis with emphasis on quality of life assessment. Dermatol Clin. 1996; 14:485–496.
Article3. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009; 361:496–509.
Article4. Sampson HA. Atopic dermatitis. Ann Allergy. 1992; 69:469–479.5. Leung DY. Atopic dermatitis: immunobiology and treatment with immune modulators. Clin Exp Immunol. 1997; 107:Suppl 1. 25–30.6. Leung DY, Soter NA. Cellular and immunologic mechanisms in atopic dermatitis. J Am Acad Dermatol. 2001; 44:1 Suppl. S1–S12.
Article7. Blanck TJ, Peterkofsky B. The stimulation of collagen secretion by ascorbate as a result of increased proline hydroxylation in chick embryo fibroblasts. Arch Biochem Biophys. 1975; 171:259–267.
Article8. Yang JH, Lee SY, Han YS, Park KC, Choy JH. Efficient transdermal penetration and improved stability of L-ascorbic acid encapsulated in an inorganic nanocapsule. Bull Korean Chem Soc. 2003; 24:499–503.
Article9. Kaur IP, Kapila M, Agrawal R. Role of novel delivery systems in developing topical antioxidants as therapeutics to combat photoageing. Ageing Res Rev. 2007; 6:271–288.
Article10. Choy JH, Kwak SY, Park JS, Jeong YJ, Portier J. Intercalative nanohybrids of nucleoside monophosphates and DNA in layered metal hydroxide. J Am Chem Soc. 1999; 121:1399–1400.
Article11. Isaacson PG. Update on MALT lymphomas. Best Pract Res Clin Haematol. 2005; 18:57–68.
Article12. Engelberts I, Möller A, Schoen GJ, van der Linden CJ, Buurman WA. Evaluation of measurement of human TNF in plasma by ELISA. Lymphokine Cytokine Res. 1991; 10:69–76.13. Harper EG, Guo C, Rizzo H, Lillis JV, Kurtz SE, Skorcheva I, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009; 129:2175–2183.
Article14. Mabuchi T, Takekoshi T, Hwang ST. Epidermal CCR6+ γδ T cells are major producers of IL-22 and IL-17 in a murine model of psoriasiform dermatitis. J Immunol. 2011; 187:5026–5031.
Article15. Maeda S, Hayami Y, Naniwa T, Ueda R. The Th17/IL-23 axis and natural immunity in psoriatic arthritis. Int J Rheumatol. 2012; 2012:539683.
Article16. Johansen C, Kragballe K, Westergaard M, Henningsen J, Kristiansen K, Iversen L. The mitogen-activated protein kinases p38 and ERK1/2 are increased in lesional psoriatic skin. Br J Dermatol. 2005; 152:37–42.
Article17. Wang S, Uchi H, Hayashida S, Urabe K, Moroi Y, Furue M. Differential expression of phosphorylated extracellular signal-regulated kinase 1/2, phosphorylated p38 mitogenactivated protein kinase and nuclear factor-kappaB p105/p50 in chronic inflammatory skin diseases. J Dermatol. 2009; 36:534–540.
Article18. Sur I, Ulvmar M, Toftgård R. The two-faced NF-kappaB in the skin. Int Rev Immunol. 2008; 27:205–223.19. Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998; 38:97–120.
Article20. Halliwell B, Gutteridge JM, Cross CE. Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med. 1992; 119:598–620.21. Xhauflaire-Uhoda E, Henry F, Piérard-Franchimont C, Piérard GE. Electrometric assessment of the effect of a zinc oxide paste in diaper dermatitis. Int J Cosmet Sci. 2009; 31:369–374.
Article22. Lansdown AB, Mirastschijski U, Stubbs N, Scanlon E, Agren MS. Zinc in wound healing: theoretical, experimental, and clinical aspects. Wound Repair Regen. 2007; 15:2–16.
Article23. Michaëlsson G, Ljunghall K. Patients with dermatitis herpetiformis, acne, psoriasis and Darier's disease have low epidermal zinc concentrations. Acta Derm Venereol. 1990; 70:304–308.24. Austin LM, Ozawa M, Kikuchi T, Walters IB, Krueger JG. The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999; 113:752–759.
Article25. Grossman RM, Krueger J, Yourish D, Granelli-Piperno A, Murphy DP, May LT, et al. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci U S A. 1989; 86:6367–6371.
Article26. Degiulio R, Montemartini C, Mazzone A, Pasotti D, Donadini A, Ricevuti G. Increased levels of leukotriene B4 and interleukin-8 in psoriatic skin. Ann N Y Acad Sci. 1993; 685:614–617.27. Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998; 111:645–649.
Article28. Fujita H, Shemer A, Suárez-Fariñas M, Johnson-Huang LM, Tintle S, Cardinale I, et al. Lesional dendritic cells in patients with chronic atopic dermatitis and psoriasis exhibit parallel ability to activate T-cell subsets. J Allergy Clin Immunol. 2011; 128:574–582.
Article29. Ono S, Otsuka A, Miyachi Y, Kabashima K. Severe psoriasis vulgaris exhibiting a high serum thymus and activationregulated chemokine level: possible association of T-helper 2 conditions. J Dermatol. 2013; 40:582–583.
Article30. Yalçin B, Tezel GG, Arda N, Erman M, Alli N. Vascular endothelial growth factor, vascular endothelial growth factor receptor-3 and cyclooxygenase-2 expression in psoriasis. Anal Quant Cytol Histol. 2007; 29:358–364.31. Roh NK, Han SH, Youn HJ, Kim YR, Lee YW, Choe YB, et al. Tissue and serum inflammatory cytokine levels in Korean psoriasis patients: a comparison between plaque and guttate psoriasis. Ann Dermatol. 2015; 27:738–743.
Article32. Takahashi H, Ibe M, Nakamura S, Ishida-Yamamoto A, Hashimoto Y, Iizuka H. Extracellular regulated kinase and c-Jun N-terminal kinase are activated in psoriatic involved epidermis. J Dermatol Sci. 2002; 30:94–99.
Article33. Haase I, Hobbs RM, Romero MR, Broad S, Watt FM. A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis. J Clin Invest. 2001; 108:527–536.
Article34. Faurschou A, Gniadecki R. TNF-alpha stimulates Akt by a distinct aPKC-dependent pathway in premalignant keratinocytes. Exp Dermatol. 2008; 17:992–997.
Article35. Lee CS, Lee SA, Kim YJ, Seo SJ, Lee MW. 3,4,5-Tricaffeoylquinic acid inhibits tumor necrosis factor-α-stimulated production of inflammatory mediators in keratinocytes via suppression of Akt- and NF-κB-pathways. Int Immunopharmacol. 2011; 11:1715–1723.
Article36. Pastore S, Mascia F, Mariotti F, Dattilo C, Mariani V, Girolomoni G. ERK1/2 regulates epidermal chemokine expression and skin inflammation. J Immunol. 2005; 174:5047–5056.
Article37. Sung YY, Kim YS, Kim HK. Illicium verum extract inhibits TNF-α- and IFN-γ-induced expression of chemokines and cytokines in human keratinocytes. J Ethnopharmacol. 2012; 144:182–189.
Article38. Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta. 2010; 1799:775–787.
Article39. Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet. 2009; 41:199–204.
Article40. Sampson HA, Albergo R. Comparison of results of skin tests, RAST, and double-blind, placebo-controlled food challenges in children with atopic dermatitis. J Allergy Clin Immunol. 1984; 74:26–33.
Article41. Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010; 11:289–293.
Article42. Hamid Q, Boguniewicz M, Leung DY. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 1994; 94:870–876.
Article43. Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001; 2:1126–1132.
Article44. Sivaranjani N, Rao SV, Rajeev G. Role of reactive oxygen species and antioxidants in atopic dermatitis. J Clin Diagn Res. 2013; 7:2683–2685.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two Cases of Delayed Skin Reaction by Sea Urchin
- Capsaicin-induced Mast Cell Activation
- Differential Effects of Digoxin on Imiquimod-Induced Psoriasis-Like Skin Inflammation on the Ear and Back
- The Role of Plant Extracts in Alleviating Particulate Matter-induced Inflammation in Barrier-interrupted Skin
- Exogenous rhTRX reduces lipid accumulation under LPS-induced inflammation