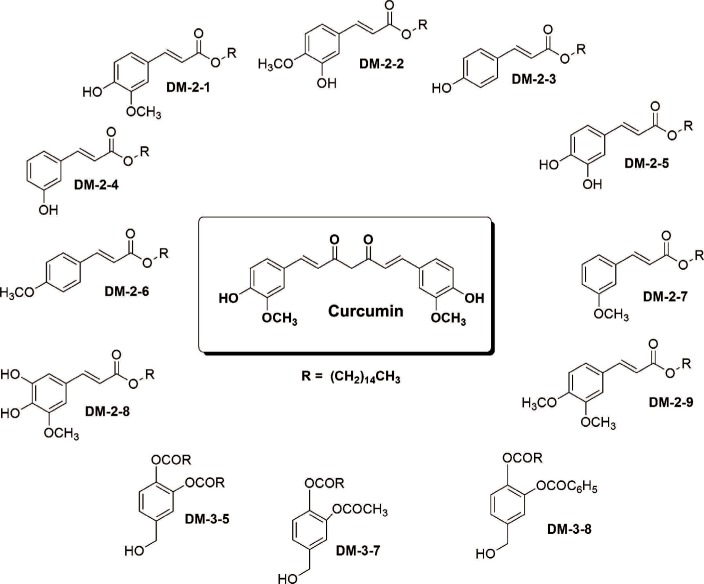
J Breast Cancer.
2016 Dec;19(4):358-371. 10.4048/jbc.2016.19.4.358.
Alkyl Cinnamates Induce Protein Kinase C Translocation and Anticancer Activity against Breast Cancer Cells through Induction of the Mitochondrial Pathway of Apoptosis
- Affiliations
-
- 1Malaria Research Group, Department of Biosciences and Bioengineering, Indian Institute of Technology Guwahati, Guwahati, India. vtrivedi@iitg.ernet.in vishalash_1999@yahoo.com
- 2Laboratory of Biological Chemistry, Department of Chemistry, Indian Institute of Technology Guwahati, Guwahati, India.
- KMID: 2362922
- DOI: http://doi.org/10.4048/jbc.2016.19.4.358
Abstract
- PURPOSE
The protein kinase C (PKC) family of serine-threonine kinases plays an important role in cancer cell progression. Thus, molecules that target PKC have potential as anticancer agents. The current study aims to understand the treatment of breast cancer cells with alkyl cinnamates. We have also explored the mechanistic details of their anticancer action and the underlying molecular signaling.
METHODS
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to measure the viability of MDAMB-231 breast cancer cells to assess the anticancer activity of these compounds. In addition, flow cytometry was performed to study the effect of alkyl cinnamates on the cell cycle and apoptosis. Immunoblotting and immunofluorescence techniques were performed to study PKC translocation, cytochrome c release, and modulation of the mitochondrial membrane potential in breast cancer cells targeted with alkyl cinnamates.
RESULTS
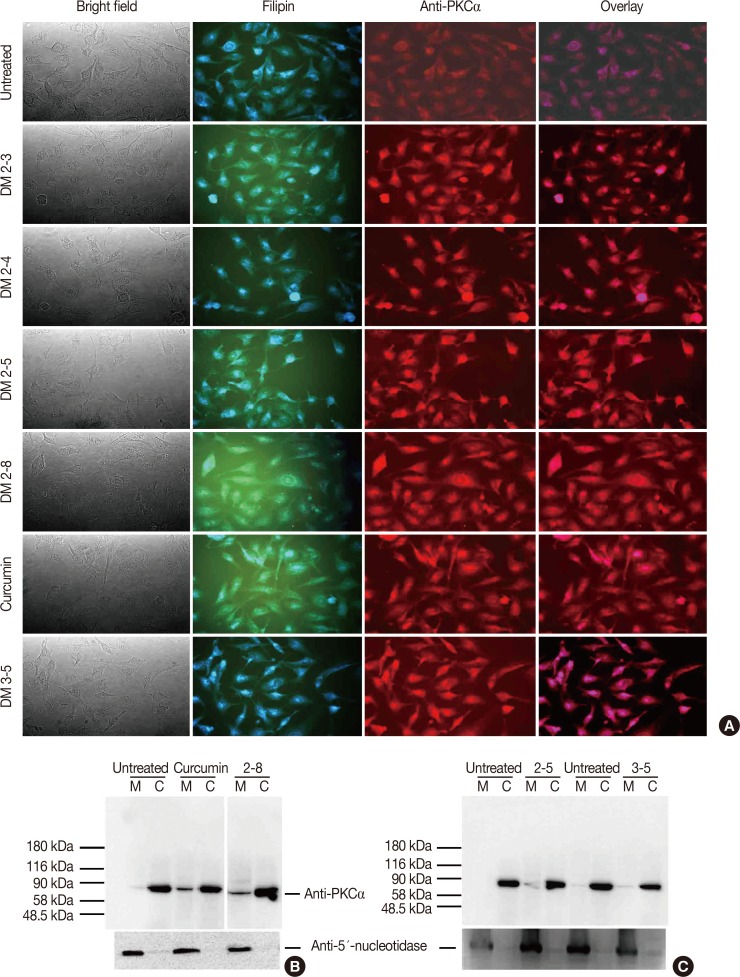
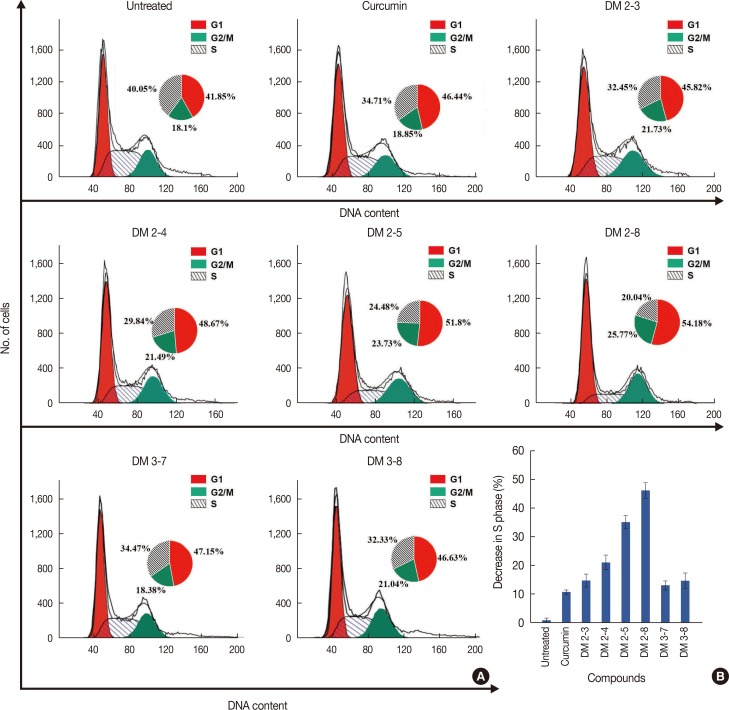
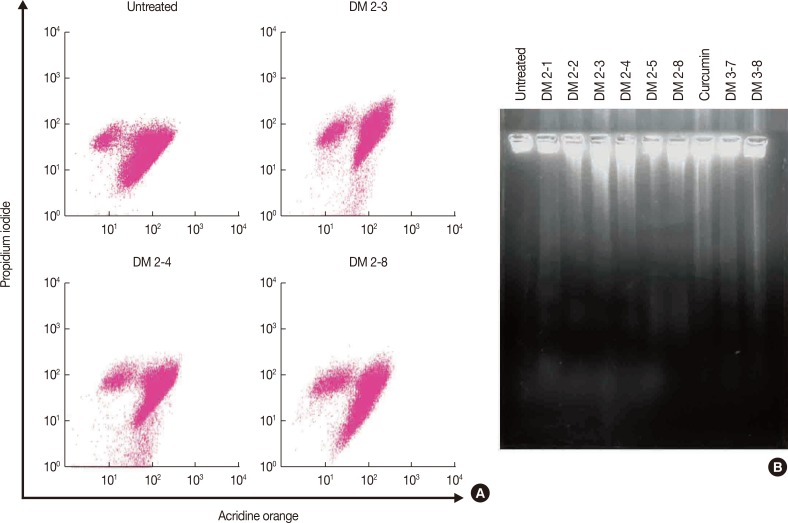
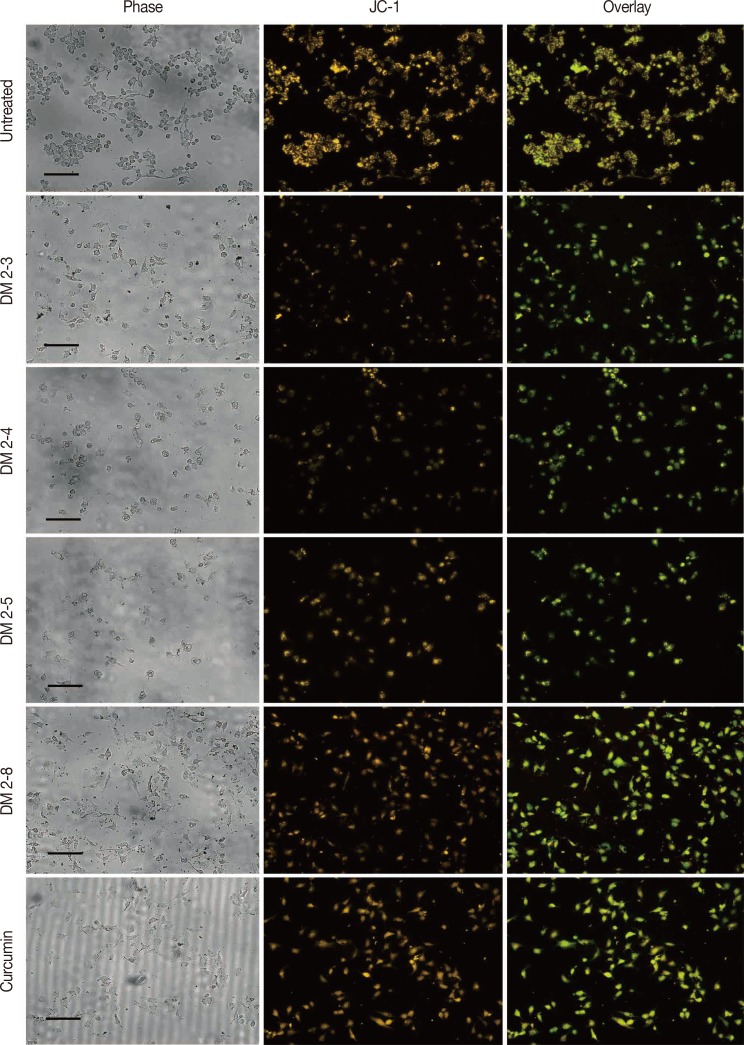
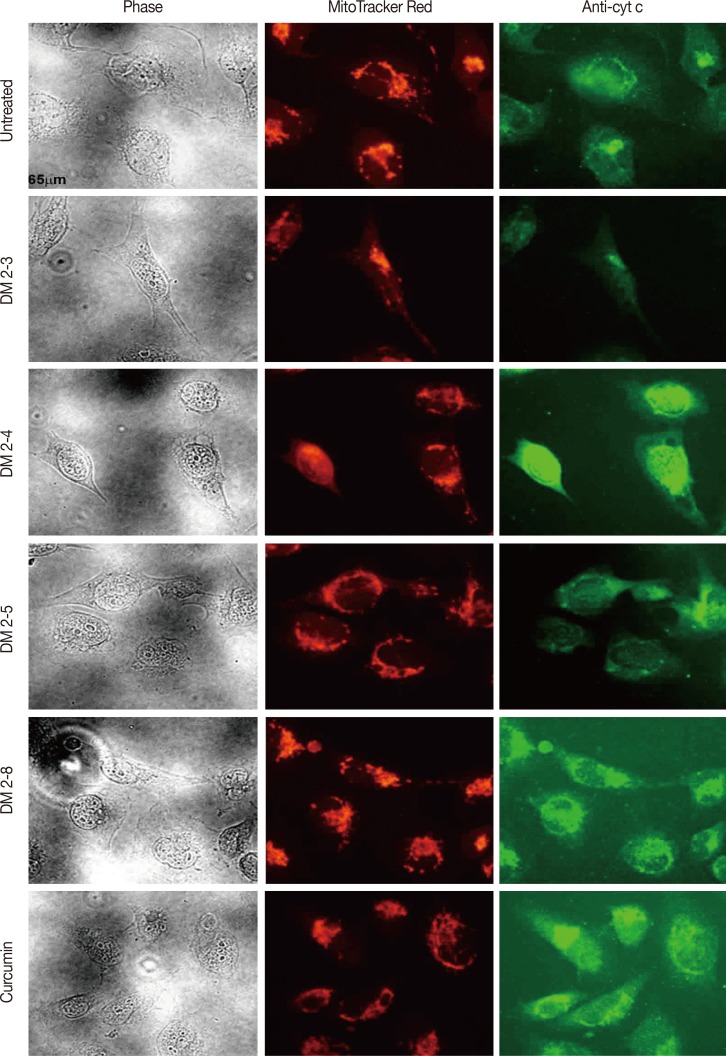
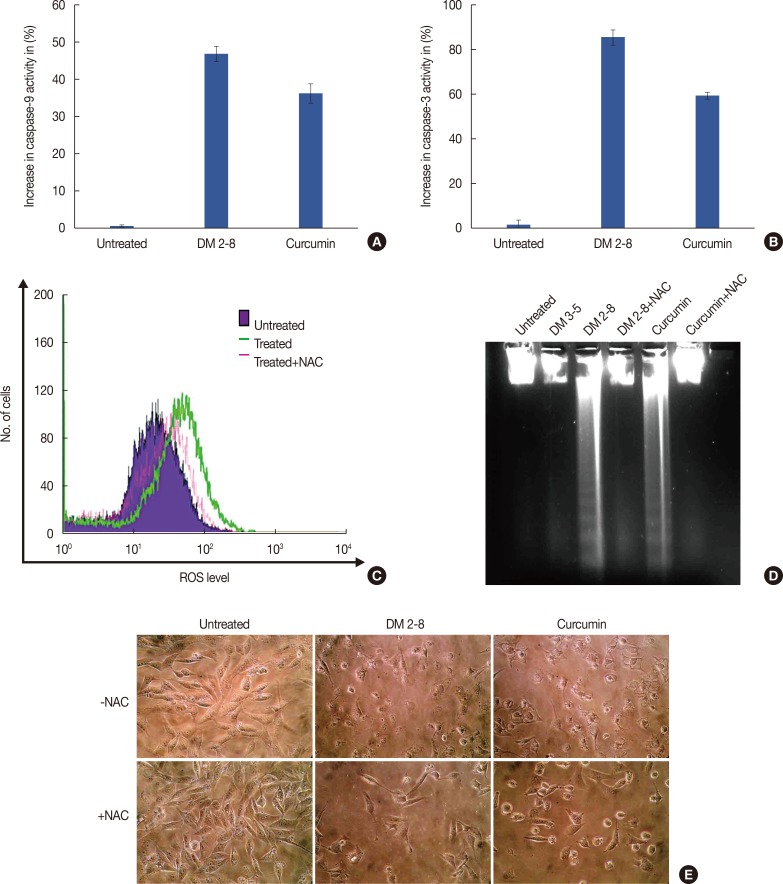
The PKC agonist DM-2-8 translocated 16.6%±1.7% PKCα from cytosol to the plasma membrane and showed excellent anticancer activity with an half maximal inhibitory concentration (IC50) of 4.13±0.27 µg/mL against cancer cells. The treated cells had an abnormal morphology and exhibited cell cycle defects with G2/M arrest and reduced S phase. Cancer cells treated with DM-2-3, DM-2-4, or DM-2-8 underwent apoptosis as the major pathway of cell death, further confirmed by genomic DNA fragmentation. Furthermore, the mitochondrial membrane potential was perturbed, indicating involvement of the mitochondrial pathway of apoptosis. Immunolocalization studies revealed cytochrome c release from mitochondria to cytosol. Cancer cells treated with DM-2-8 and curcumin showed activation of caspase-9 and caspase-3 as downstream molecular components of the apoptotic pathway. Alkyl cinnamates also caused oxidative stress, which regulates the apoptotic machinery (DNA fragmentation), cell death, and morphological abnormalities in cancer cells.
CONCLUSION
Alkyl cinnamates specifically target cancer cells through induction of PKC translocation and the mitochondrial pathway of apoptosis, and could be promising anticancer drugs.
Keyword
MeSH Terms
-
Antineoplastic Agents
Apoptosis*
Breast Neoplasms*
Breast*
Caspase 3
Caspase 9
Caspases
Cell Cycle
Cell Death
Cell Membrane
Cinnamates*
Curcumin
Cytochromes c
Cytosol
DNA Fragmentation
Flow Cytometry
Fluorescent Antibody Technique
Humans
Immunoblotting
Membrane Potential, Mitochondrial
Mitochondria
Oxidative Stress
Protein Kinase C*
Protein Kinases*
Protein-Serine-Threonine Kinases
S Phase
Antineoplastic Agents
Caspase 3
Caspase 9
Caspases
Cinnamates
Curcumin
Cytochromes c
Protein Kinase C
Protein Kinases
Protein-Serine-Threonine Kinases
Figure
Reference
-
2. Koivunen J, Aaltonen V, Peltonen J. Protein kinase C (PKC) family in cancer progression. Cancer Lett. 2006; 235:1–10. PMID: 15907369.
Article3. Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007; 7:281–294. PMID: 17384583.
Article4. O'Brian C, Vogel VG, Singletary SE, Ward NE. Elevated protein kinase C expression in human breast tumor biopsies relative to normal breast tissue. Cancer Res. 1989; 49:3215–3217. PMID: 2720675.5. Powell CT, Brittis NJ, Stec D, Hug H, Heston WD, Fair WR. Persistent membrane translocation of protein kinase C alpha during 12-0-tetradecanoylphorbol-13-acetate-induced apoptosis of LNCaP human prostate cancer cells. Cell Growth Differ. 1996; 7:419–428. PMID: 9052983.6. Wu TT, Hsieh YH, Hsieh YS, Liu JY. Reduction of PKC alpha decreases cell proliferation, migration, and invasion of human malignant hepatocellular carcinoma. J Cell Biochem. 2008; 103:9–20. PMID: 17486587.7. Tanaka Y, Gavrielides MV, Mitsuuchi Y, Fujii T, Kazanietz MG. Protein kinase C promotes apoptosis in LNCaP prostate cancer cells through activation of p38 MAPK and inhibition of the Akt survival pathway. J Biol Chem. 2003; 278:33753–33762. PMID: 12824193.
Article8. Chen ZF, Fang JY, Weng YR, Sun DF, Wang X, Lu R. The effect of PKC-delta inhibitor rottlerin on human colon cancer cell line SW1116 and its mechanism. Zhonghua Zhong Liu Za Zhi. 2006; 28:564–567. PMID: 17236547.9. Santiago-Walker AE, Fikaris AJ, Kao GD, Brown EJ, Kazanietz MG, Meinkoth JL. Protein kinase C delta stimulates apoptosis by initiating G1 phase cell cycle progression and S phase arrest. J Biol Chem. 2005; 280:32107–32114. PMID: 16051606.10. Ferriola PC, Cody V, Middleton E Jr. Protein kinase C inhibition by plant flavonoids. Kinetic mechanisms and structure-activity relationships. Biochem Pharmacol. 1989; 38:1617–1624. PMID: 2730676.11. Yang EB, Zhao YN, Zhang K, Mack P. Daphnetin, one of coumarin derivatives, is a protein kinase inhibitor. Biochem Biophys Res Commun. 1999; 260:682–685. PMID: 10403826.
Article12. Aitken A. The activation of protein kinase C by daphnane, ingenane and tigliane diterpenoid esters. Bot J Linn Soc. 1987; 94:247–263.
Article13. Mamidi N, Gorai S, Sahoo J, Manna D. Alkyl cinnamates as regulator for the C1 domain of protein kinase C isoforms. Chem Phys Lipids. 2012; 165:320–330. PMID: 22414757.
Article14. Trivedi V, Zhang SC, Castoreno AB, Stockinger W, Shieh EC, Vyas JM, et al. Immunoglobulin G signaling activates lysosome/phagosome docking. Proc Natl Acad Sci U S A. 2006; 103:18226–18231. PMID: 17110435.
Article15. Deshmukh R, Trivedi V. Phagocytic uptake of oxidized heme polymer is highly cytotoxic to macrophages. PLoS One. 2014; 9:e103706. PMID: 25078090.
Article16. Trivedi V, Chand P, Srivastava K, Puri SK, Maulik PR, Bandyopadhyay U. Clotrimazole inhibits hemoperoxidase of Plasmodium falciparum and induces oxidative stress: proposed antimalarial mechanism of clotrimazole. J Biol Chem. 2005; 280:41129–41136. PMID: 15863504.17. Black AR, Black JD. Protein kinase C signaling and cell cycle regulation. Front Immunol. 2013; 3:423. PMID: 23335926.
Article18. Xue X, Yu JL, Sun DQ, Kong F, Qu XJ, Zou W, et al. Curcumin induces apoptosis in SGC-7901 gastric adenocarcinoma cells via regulation of mitochondrial signaling pathways. Asian Pac J Cancer Prev. 2014; 15:3987–3992. PMID: 24935585.
Article19. Wender PA, Baryza JL, Brenner SE, DeChristopher BA, Loy BA, Schrier AJ, et al. Design, synthesis, and evaluation of potent bryostatin analogs that modulate PKC translocation selectivity. Proc Natl Acad Sci U S A. 2011; 108:6721–6726. PMID: 21415363.
Article20. Géczy T, Oláh A, Tóth BI, Czifra G, Szöllősi AG, Szabó T, et al. Protein kinase C isoforms have differential roles in the regulation of human sebocyte biology. J Invest Dermatol. 2012; 132:1988–1997. PMID: 22475757.
Article21. Frey MR, Saxon ML, Zhao X, Rollins A, Evans SS, Black JD. Protein kinase C isozyme-mediated cell cycle arrest involves induction of p21(waf1/cip1) and p27(kip1) and hypophosphorylation of the retinoblastoma protein in intestinal epithelial cells. J Biol Chem. 1997; 272:9424–9435. PMID: 9083081.22. Gutcher I, Webb PR, Anderson NG. The isoform-specific regulation of apoptosis by protein kinase C. Cell Mol Life Sci. 2003; 60:1061–1070. PMID: 12861375.
Article23. Garcia-Bermejo ML, Leskow FC, Fujii T, Wang Q, Blumberg PM, Ohba M, et al. Diacylglycerol (DAG)-lactones, a new class of protein kinase C (PKC) agonists, induce apoptosis in LNCaP prostate cancer cells by selective activation of PKCalpha. J Biol Chem. 2002; 277:645–655. PMID: 11584014.24. Clark JA, Black AR, Leontieva OV, Frey MR, Pysz MA, Kunneva L, et al. Involvement of the ERK signaling cascade in protein kinase C-mediated cell cycle arrest in intestinal epithelial cells. J Biol Chem. 2004; 279:9233–9247. PMID: 14670956.
Article25. Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011; 30:87. PMID: 21943236.
Article26. Tong QS, Zheng LD, Lu P, Jiang FC, Chen FM, Zeng FQ, et al. Apoptosis-inducing effects of curcumin derivatives in human bladder cancer cells. Anticancer Drugs. 2006; 17:279–287. PMID: 16520656.
Article27. Subramaniam D, May R, Sureban SM, Lee KB, George R, Kuppusamy P, et al. Diphenyl difluoroketone: a curcumin derivative with potent in vivo anticancer activity. Cancer Res. 2008; 68:1962–1969. PMID: 18339878.
Article28. Sun A, Lu YJ, Hu H, Shoji M, Liotta DC, Snyder JP. Curcumin analog cytotoxicity against breast cancer cells: exploitation of a redox-dependent mechanism. Bioorg Med Chem Lett. 2009; 19:6627–6631. PMID: 19854644.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Synthetic Chenodeoxycholic Acid Derivative HS-1200-induced Apoptosis of Human Melanoma Cells
- Activation of SAPK and increase in Bak levels during ceramide and indomethacin-induced apoptosis in HT29 cells
- Recombinant Rv0753c Protein of Mycobacterium tuberculosis Induces Apoptosis Through Reactive Oxygen Species-JNK Pathway in Macrophages
- Induction of cytoprotective autophagy by morusin via AMP-activated protein kinase activation in human non-small cell lung cancer cells
- Expression Status and Prognostic Value of bcl-2 Protein in Breast Cancer