Nutr Res Pract.
2015 Apr;9(2):129-136. 10.4162/nrp.2015.9.2.129.
Active hexose correlated compound potentiates the antitumor effects of low-dose 5-fluorouracil through modulation of immune function in hepatoma 22 tumor-bearing mice
- Affiliations
-
- 1Fujian Academy of Integrative Medicine, Fujian University of Traditional Chinese Medicine, Huatuo Road, No1, Fuzhou, 350108, China. llm@fjtcm.edu.cn
- 2The Second People's Hospital of Fujian Province, China.
- 3Inspection and Quarantine Technique Centre of Fujian Entry-exit Inspection and Quarantine Bureau, China.
- KMID: 2313820
- DOI: http://doi.org/10.4162/nrp.2015.9.2.129
Abstract
- BACKGROUND/OBJECTIVES
A variety of immunomodulators can improve the efficacy of low-dose chemotherapeutics. Active hexose correlated compound (AHCC), a mushroom mycelia extract, has been shown to be a strong immunomodulator. Whether AHCC could enhance the antitumor effect of low-dose 5-fluorouracil (5-FU) via regulation of host immunity is unknown.
MATERIALS/METHODS
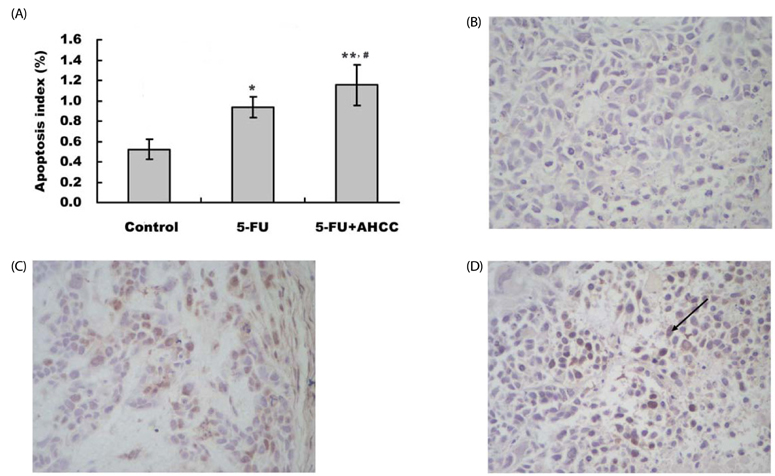
In the current study Hepatoma 22 (H22) tumor-bearing mice were treated with PBS, 5-FU (10 mg.kg-1.d-1, i.p), or AHCC (360 mg.kg-1.d-1, i.g) plus 5-FU, respectively, for 5 d. CD3+, CD4+, CD8+, and NK in peripheral blood were detected by flow cytometry. ALT, AST, BUN, and Cr levels were measured by biochemical assay. IL-2 and TNFalpha in serum were measured using the RIA kit and apoptosis of tumor was detected by TUNEL staining. Bax, Bcl-2, and TS protein levels were measured by immunohistochemical staining and mRNA level was evaluated by RT-PCR.
RESULTS
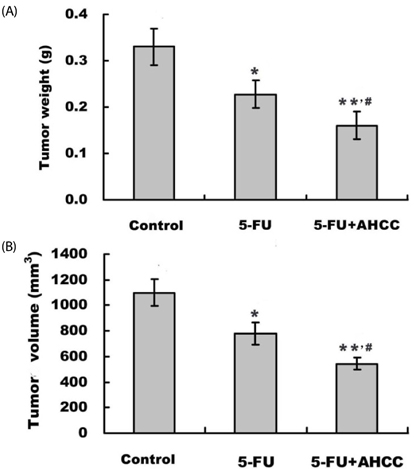
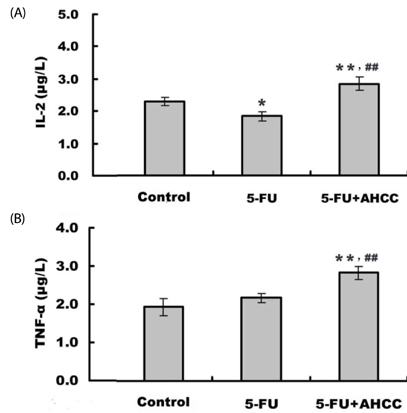
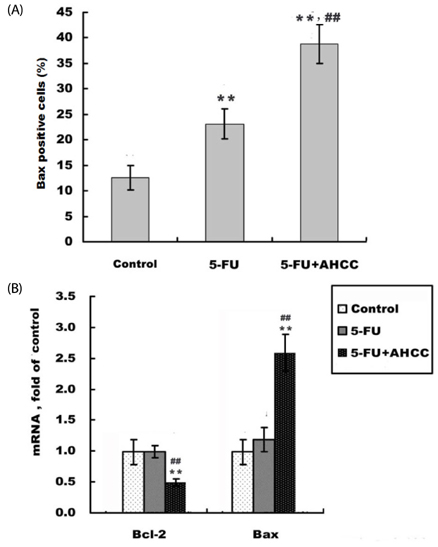
Diet consumption and body weight showed that AHCC had no apparent toxicity. AHCC could reverse liver injury and myelosuppression induced by 5-FU (P < 0.05). Compared to mice treated with 5-FU, mice treated with AHCC plus 5-FU had higher thymus index, percentages of CD3+, CD4+, and NK cells (P < 0.01), and ratio of CD4+/CD8+ (P < 0.01) in peripheral blood. Radioimmunoassay showed that mice treated with AHCC plus 5-FU had the highest serum levels of IL-2 and TNFalpha compared with the vehicle group and 5-FU group. More importantly, the combination of AHCC and 5-FU produced a more potent antitumor effect (P < 0.05) and caused more severe apoptosis in tumor tissue (P < 0.05) compared with the 5-FU group. In addition, the combination of AHCC and 5-FU further up-regulated the expression of Bcl-2 associated X protein (Bax) (P < 0.01), while it down-regulated the expression of B cell lymphoma 2 (Bcl-2) (P < 0.01).
CONCLUSIONS
These results support the claim that AHCC might be beneficial for cancer patients receiving chemotherapy.
Keyword
MeSH Terms
-
Agaricales
Animals
Apoptosis
Body Weight
Carcinoma, Hepatocellular*
Diet
Drug Therapy
Flow Cytometry
Fluorouracil*
Humans
Immunologic Factors
In Situ Nick-End Labeling
Interleukin-2
Killer Cells, Natural
Liver
Lymphoma, B-Cell
Mice*
Radioimmunoassay
RNA, Messenger
Thymus Gland
Tumor Necrosis Factor-alpha
Fluorouracil
Immunologic Factors
Interleukin-2
RNA, Messenger
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Kidd PM. The use of mushroom glucans and proteoglycans in cancer treatment. Altern Med Rev. 2000; 5:4–27.2. Burikhanov RB, Wakame K, Igarashi Y, Wang S, Matsuzaki S. Suppressive effect of active hexose correlated compound (AHCC) on thymic apoptosis induced by dexamethasone in the rat. Endocr Regul. 2000; 34:181–188.3. Aviles H, Belay T, Vance M, Sun B, Sonnenfeld G. Active hexose correlated compound enhances the immune function of mice in the hindlimb-unloading model of spaceflight conditions. J Appl Physiol (1985). 2004; 97:1437–1444.
Article4. Uno K, Kosuna K, Sun B, Fujii H, Wakame K, Chikumaru S, Hosokawa G, Ueda Y. Active hexose correlated compound (AHCC) improves immunological parameters and performance status of patients with solid tumors. Biotherapy (Tokyo). 2000; 14:303–309.5. Aviles H, O'Donnell P, Orshal J, Fujii H, Sun B, Sonnenfeld G. Active hexose correlated compound activates immune function to decrease bacterial load in a murine model of intramuscular infection. Am J Surg. 2008; 195:537–545.
Article6. Nogusa S, Gerbino J, Ritz BW. Low-dose supplementation with active hexose correlated compound improves the immune response to acute influenza infection in C57BL/6 mice. Nutr Res. 2009; 29:139–143.
Article7. Ritz BW, Nogusa S, Ackerman EA, Gardner EM. Supplementation with active hexose correlated compound increases the innate immune response of young mice to primary influenza infection. J Nutr. 2006; 136:2868–2873.
Article8. Wang S, Welte T, Fang H, Chang GJ, Born WK, O'Brien RL, Sun B, Fujii H, Kosuna K, Wang T. Oral administration of active hexose correlated compound enhances host resistance to West Nile encephalitis in mice. J Nutr. 2009; 139:598–602.
Article9. Matsui Y, Uhara J, Satoi S, Kaibori M, Yamada H, Kitade H, Imamura A, Takai S, Kawaguchi Y, Kwon AH, Kamiyama Y. Improved prognosis of postoperative hepatocellular carcinoma patients when treated with functional foods: a prospective cohort study. J Hepatol. 2002; 37:78–86.
Article10. Cowawintaweewat S, Manoromana S, Sriplung H, Khuhaprema T, Tongtawe P, Tapchaisri P, Chaicumpa W. Prognostic improvement of patients with advanced liver cancer after active hexose correlated compound (AHCC) treatment. Asian Pac J Allergy Immunol. 2006; 24:33–45.11. Casale F, Canaparo R, Serpe L, Muntoni E, Pepa CD, Costa M, Mairone L, Zara GP, Fornari G, Eandi M. Plasma concentrations of 5-fluorouracil and its metabolites in colon cancer patients. Pharmacol Res. 2004; 50:173–179.12. Baglan KL, Frazier RC, Yan D, Huang RR, Martinez AA, Robertson JM. The dose-volume relationship of acute small bowel toxicity from concurrent 5-FU-based chemotherapy and radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2002; 52:176–183.
Article13. Dencausse Y, Sturm J, Hartung G, Dietzler P, Edler L, Bambach M, Wojatschek C, Lindemann H, Qeisser W. Adjuvant radio-chemotherapy in stage II-III rectal cancer with 24-hour infusion of high-dose 5-fluorouracil and folinic acid: evaluation of feasibility. Onkologie. 2001; 24:476–480.
Article14. Grem JL, McAtee N, Steinberg SM, Hamilton JM, Murphy RF, Drake J, Chisena T, Balis F, Cysyk R, Arbuck SG, Sorensen JM, Chen AP, Goldstein L, Jordan E, Setser A, Goldspiel B, DeCarvalho M, Allegra CJ. A phase I study of continuous infusion 5-fluorouracil plus calcium leucovorin in combination with N-(phosphonacetyl)-L-aspartate in metastatic gastrointestinal adenocarcinoma. Cancer Res. 1993; 53:4828–4836.15. Kobayashi R, Yoshimatsu K, Yokomizo H, Katsube T, Ogawa K. Low-dose chemotherapy with leucovorin plus 5-fluorouracil for colorectal cancer can maintain host immunity. Anticancer Res. 2007; 27:675–679.16. Yoshimatsu K, Yokomizo H, Fujimoto T, Umehara A, Otani T, Matsumoto A, Osawa G, Shiozawa S, Katsube T, Naritaka Y, Ogawa K. First-line chemotherapy with low-dose leucovorin plus 5-fluorouracil (LV/5-FU) for elderly patients with metastatic colorectal cancer. Anticancer Res. 2007; 27:1641–1644.17. Butterfield LH, Ribas A. Immunotherapy of hepatocellular carcinoma. Expert Opin Biol Ther. 2002; 2:123–133.
Article18. Thomas MB, Jaffe D, Choti MM, Belghiti J, Curley S, Fong Y, Gores G, Kerlan R, Merle P, O'Neil B, Poon R, Schwartz L, Tepper J, Yao F, Haller D, Mooney M, Venook A. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010; 28:3994–4005.
Article19. Burroughs A, Hochhauser D, Meyer T. Systemic treatment and liver transplantation for hepatocellular carcinoma: two ends of the therapeutic spectrum. Lancet Oncol. 2004; 5:409–418.
Article20. Ganne-Carrié N, Trinchet JC. Systemic treatment of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2004; 16:275–281.21. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003; 37:429–442.
Article22. Kasai K, Ushio A, Kasai Y, Sawara K, Miyamoto Y, Oikawa K, Kuroda H, Takikawa Y, Suzuki K. Therapeutic efficacy of combination therapy with intra-arterial 5-fluorouracil and systemic pegylated interferon α-2b for advanced hepatocellular carcinoma with portal venous invasion. Cancer. 2012; 118:3302–3310.
Article23. Nagano H, Wada H, Kobayashi S, Marubashi S, Eguchi H, Tanemura M, Tomimaru Y, Osuga K, Umeshita K, Doki Y, Mori M. Long-term outcome of combined interferon-α and 5-fluorouracil treatment for advanced hepatocellular carcinoma with major portal vein thrombosis. Oncology. 2011; 80:63–69.
Article24. Noda T, Nagano H, Takemasa I, Yoshioka S, Murakami M, Wada H, Kobayashi S, Marubashi S, Takeda Y, Dono K, Umeshita K, Matsuura N, Matsubara K, Doki Y, Mori M, Monden M. Activation of Wnt/beta-catenin signalling pathway induces chemoresistance to interferon-alpha/5-fluorouracil combination therapy for hepatocellular carcinoma. Br J Cancer. 2009; 100:1647–1658.
Article25. Wada H, Nagano H, Yamamoto H, Noda T, Murakami M, Kobayashi S, Marubashi S, Eguchi H, Takeda Y, Tanemura M, Umeshita K, Doki Y, Mori M. Combination of interferon-alpha and 5-fluorouracil inhibits endothelial cell growth directly and by regulation of angiogenic factors released by tumor cells. BMC Cancer. 2009; 9:361.
Article26. Yin H, Xie F, Zhang J, Yang Y, Deng B, Sun J, Wang Q, Qu X, Mao H. Combination of interferon-α and 5-fluorouracil induces apoptosis through mitochondrial pathway in hepatocellular carcinoma in vitro. Cancer Lett. 2011; 306:34–42.
Article27. Monden M, Sakon M, Sakata Y, Ueda Y, Hashimura E. FAIT Research Group. 5-fluorouracil arterial infusion + interferon therapy for highly advanced hepatocellular carcinoma: A multicenter, randomized, phase II study. Hepatol Res. 2012; 42:150–165.
Article28. Ghoneum M, Wimbley M, Salem F, McKlain A, Attallah N, Gill G. Immu-nomodulatory and anticancer effects of active hexose correlated compound (AHCC). Int J Immunother. 1995; 11:23–28.29. Hirose A, Sato E, Fujii H, Sun B, Nishioka H, Aruoma OI. The influence of active hexose correlated compound (AHCC) on cisplatin-evoked chemotherapeutic and side effects in tumor-bearing mice. Toxicol Appl Pharmacol. 2007; 222:152–158.
Article30. Shigama K, Nakaya A, Wakame K, Nishioka H, Fujii H. Alleviating effect of active hexose correlated compound (AHCC) for anticancer drug-induced side effects in non-tumor-bearing mice. J Exp Ther Oncol. 2009; 8:43–51.31. Sun B, Wakame K, Sato E, Nishioka H, Aruoma OI, Fujii H. The effect of active hexose correlated compound in modulating cytosine arabinoside-induced hair loss, and 6-mercaptopurine- and methotrexate-induced liver injury in rodents. Cancer Epidemiol. 2009; 33:293–299.
Article32. Hunter RJ, Fujii H, Wakame K, Gaikwad A, Wolf JK, Smith JA. Evaluation of active hexose correlated compound (AHCC) in combination with pegylated liposomal doxorubicin for treatment of ovarian cancer. Int J Appl Res Nat Prod. 2011; 4:6–14.33. Fujii H, Nishioka N, Simon RR, Kaur R, Lynch B, Roberts A. Genotoxicity and subchronic toxicity evaluation of Active Hexose Correlated Compound (AHCC). Regul Toxicol Pharmacol. 2011; 59:237–250.
Article34. Lanier LL. NK cell recognition. Annu Rev Immunol. 2005; 23:225–274.
Article35. Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976; 193:1007–1008.
Article36. Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988; 240:1169–1176.
Article37. Dillman RO. The clinical experience with interleukin-2 in cancer therapy. Cancer Biother. 1994; 9:183–209.
Article38. Ferrante A. Activation of neutrophils by interleukins-1 and -2 and tumor necrosis factors. Immunol Ser. 1992; 57:417–436.39. Zhu HG, Zollner TM, Klein-Franke A, Anderer FA. Activation of human monocyte/macrophage cytotoxicity by IL-2/IFN gamma is linked to increased expression of an antitumor receptor with specificity for acetylated mannose. Immunol Lett. 1993; 38:111–119.
Article40. Ibayashi Y, Tokuda Y, Saks ER, Sarna GP, Golub SH. In vivo and in vitro activation of NK cytotoxicity with IL-2. Prog Clin Biol Res. 1987; 244:275–285.41. Chatzidakis I, Mamalaki C. T cells as sources and targets of TNF: implications for immunity and autoimmunity. Curr Dir Autoimmun. 2010; 11:105–118.
Article42. Yagita A, Maruyama S, Wakasugi S, Sukegawa Y. H-2 haplotype-dependent serum IL-12 production in tumor-bearing mice treated with various mycelial extracts. In Vivo. 2002; 16:49–54.43. Yin Z, Fujii H, Walshe T. Effects of active hexose correlated compound on frequency of CD4+ and CD8+ T cells producing interferon-γ and/or tumor necrosis factor-α in healthy adults. Hum Immunol. 2010; 71:1187–1190.
Article44. Daddaoua A, Martínez-Plata E, López-Posadas R, Vieites JM, González M, Requena P, Zarzuelo A, Suárez MD, de Medina FS, Martínez-Augustin O. Active hexose correlated compound acts as a prebiotic and is antiinflammatory in rats with hapten-induced colitis. J Nutr. 2007; 137:1222–1228.
Article45. Li JH, Rosen D, Ronen D, Behrens CK, Krammer PH, Clark WR, Berke G. The regulation of CD95 ligand expression and function in CTL. J Immunol. 1998; 161:3943–3949.46. Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H, Okumura K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001; 7:94–100.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Aquatic Exercise at Thermoneutral Water Temperature Enhances Antitumor Immune Responses
- Effects of Active Hexose Correlated Compounds on Drug Induced Liver Injury in Mice
- Immunomodulating and Antitumor Activities of Exo-secretion from Phellinus linteus
- Stimulatory Effect of IL-10 on Antitumor Cytolytic Activity of Murine Spleen Cells
- Anti-tumor effects of Toxoplasma gondii and antigen-pulsed dendritic cells in mice bearing breast cancer