Nat Prod Sci.
2015 Dec;21(4):240-247. 10.20307/nps.2015.21.4.240.
Viridicatol from Marine-derived Fungal Strain Penicillium sp. SF-5295 Exerts Anti-inflammatory Effects through Inhibiting NF-kappaB Signaling Pathway on Lipopolysaccharide-induced RAW264.7 and BV2 Cells
- Affiliations
-
- 1College of Pharmacy, Wonkwang University, Iksan 570-749, Korea. yckim@wku.ac.kr, hoh@wku.ac.kr
- 2College of Medical and Life Sciences, Silla University, Busan 617-736, Republic of Korea.
- KMID: 2312903
- DOI: http://doi.org/10.20307/nps.2015.21.4.240
Abstract
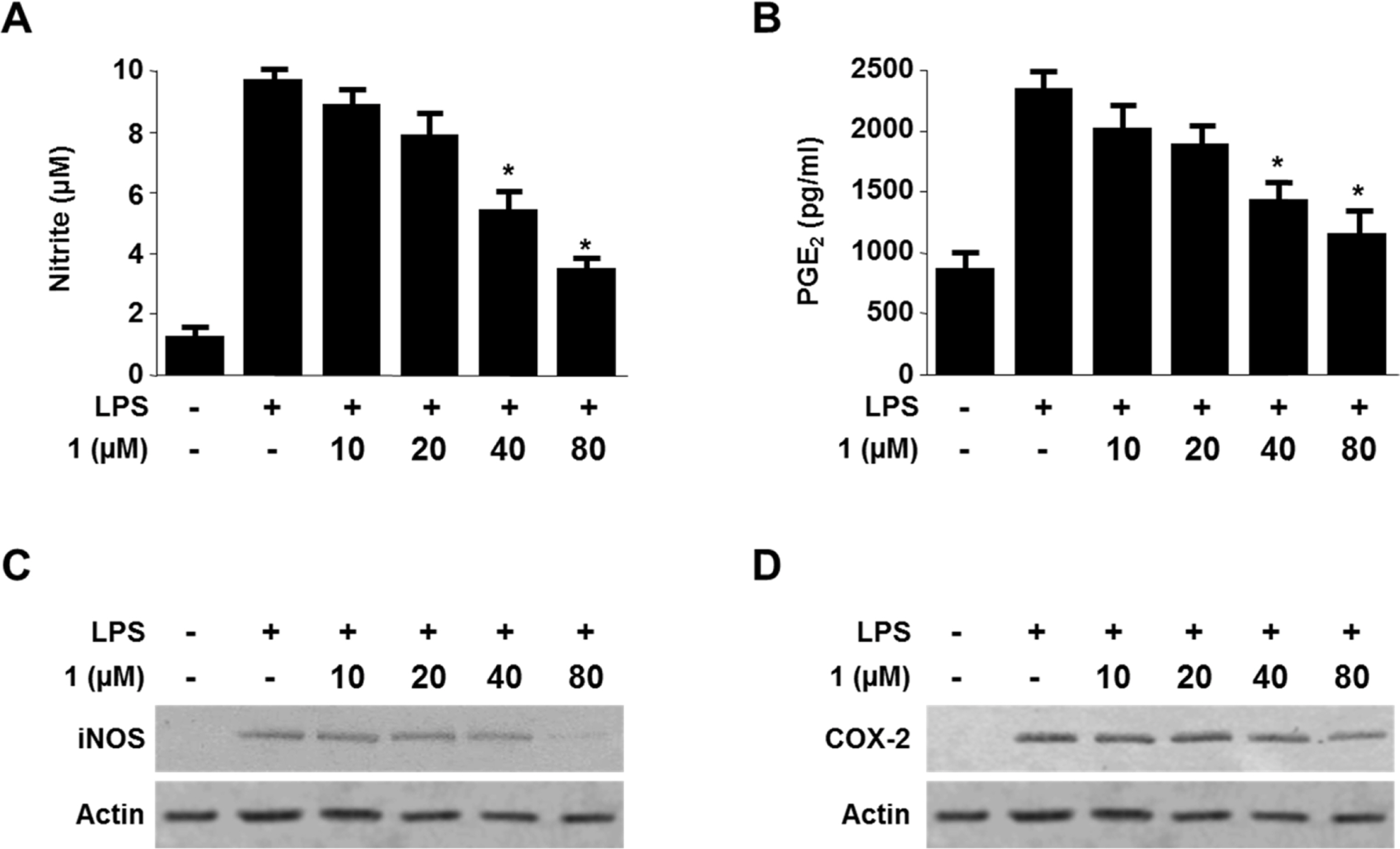
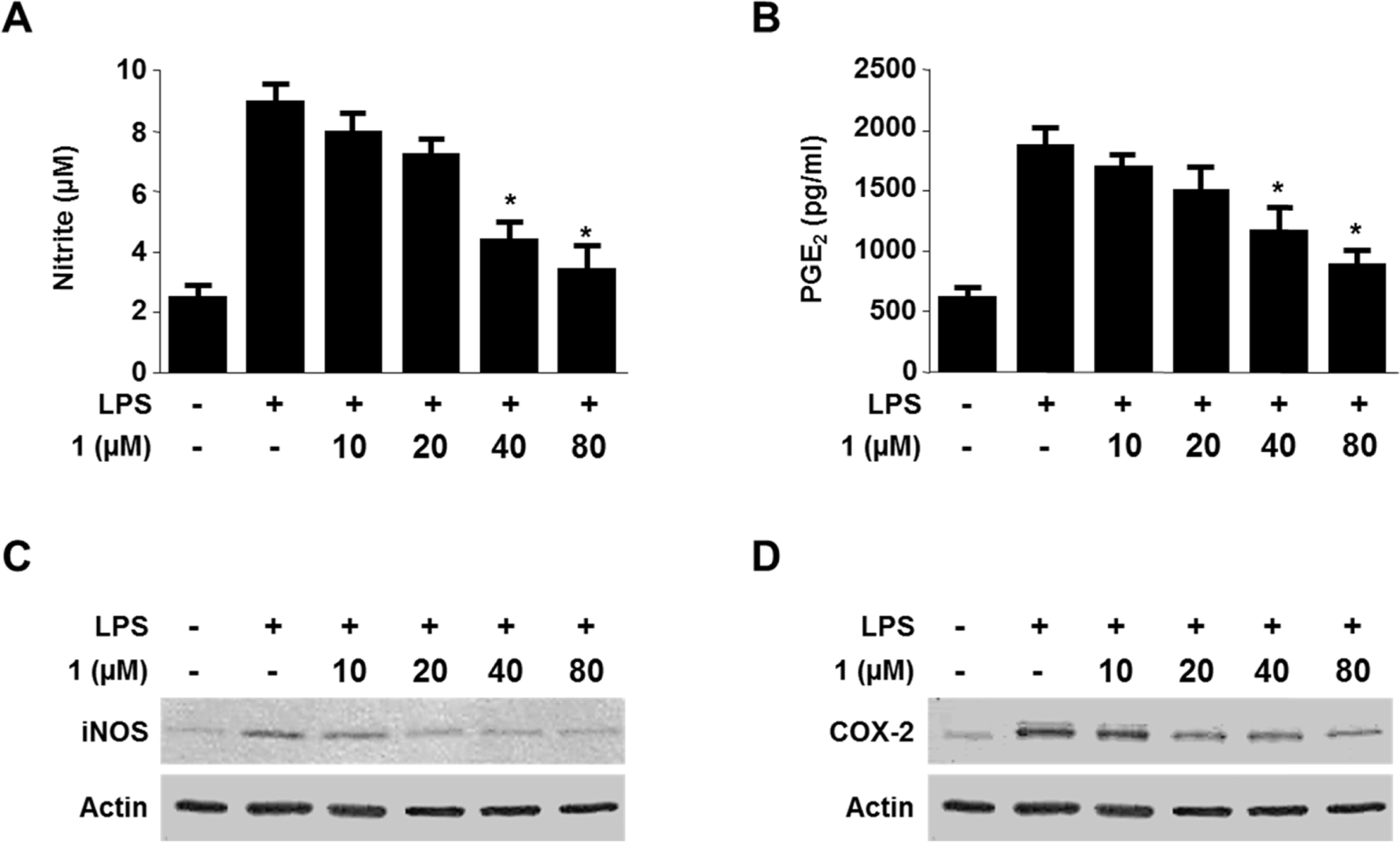
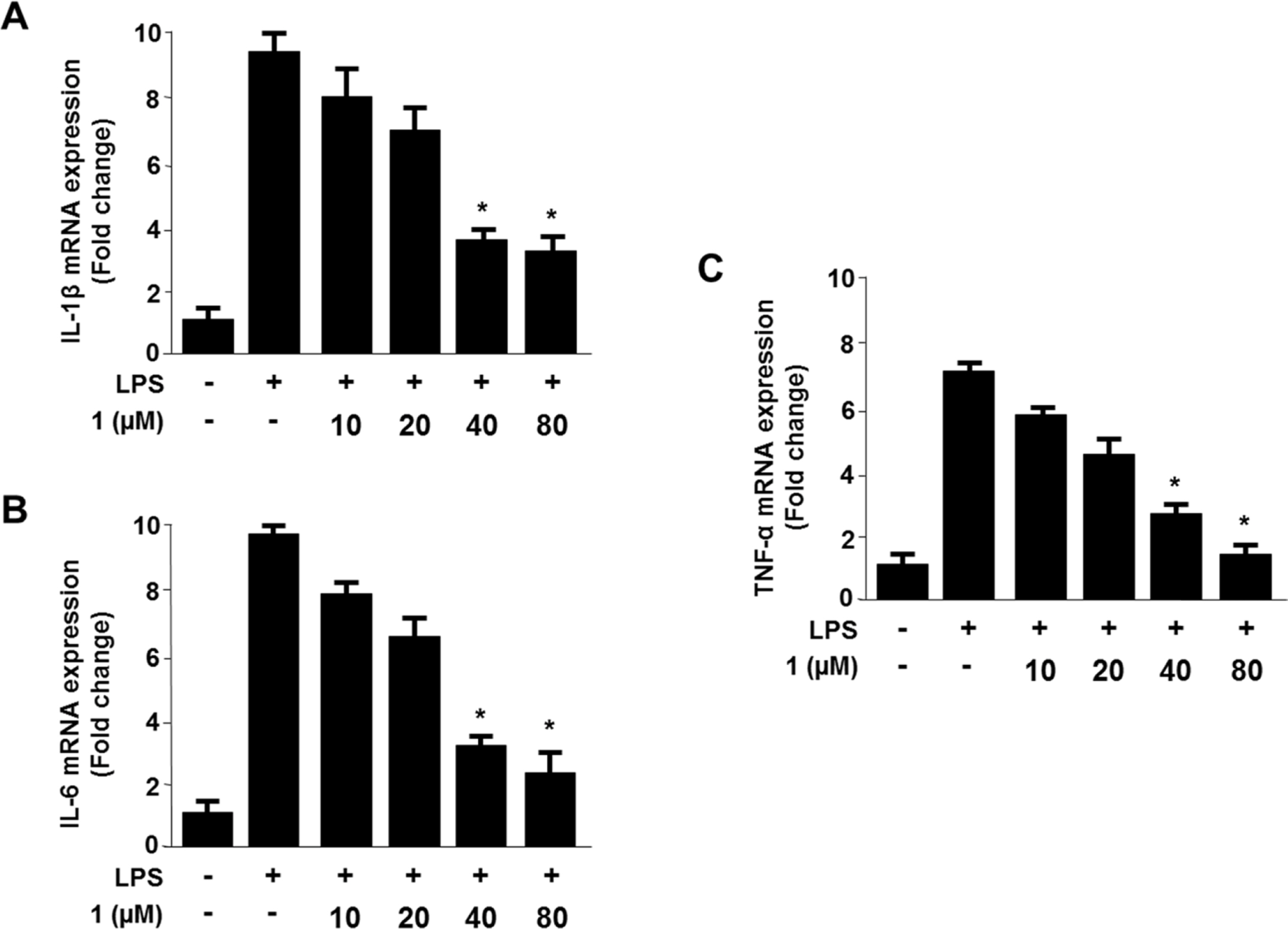
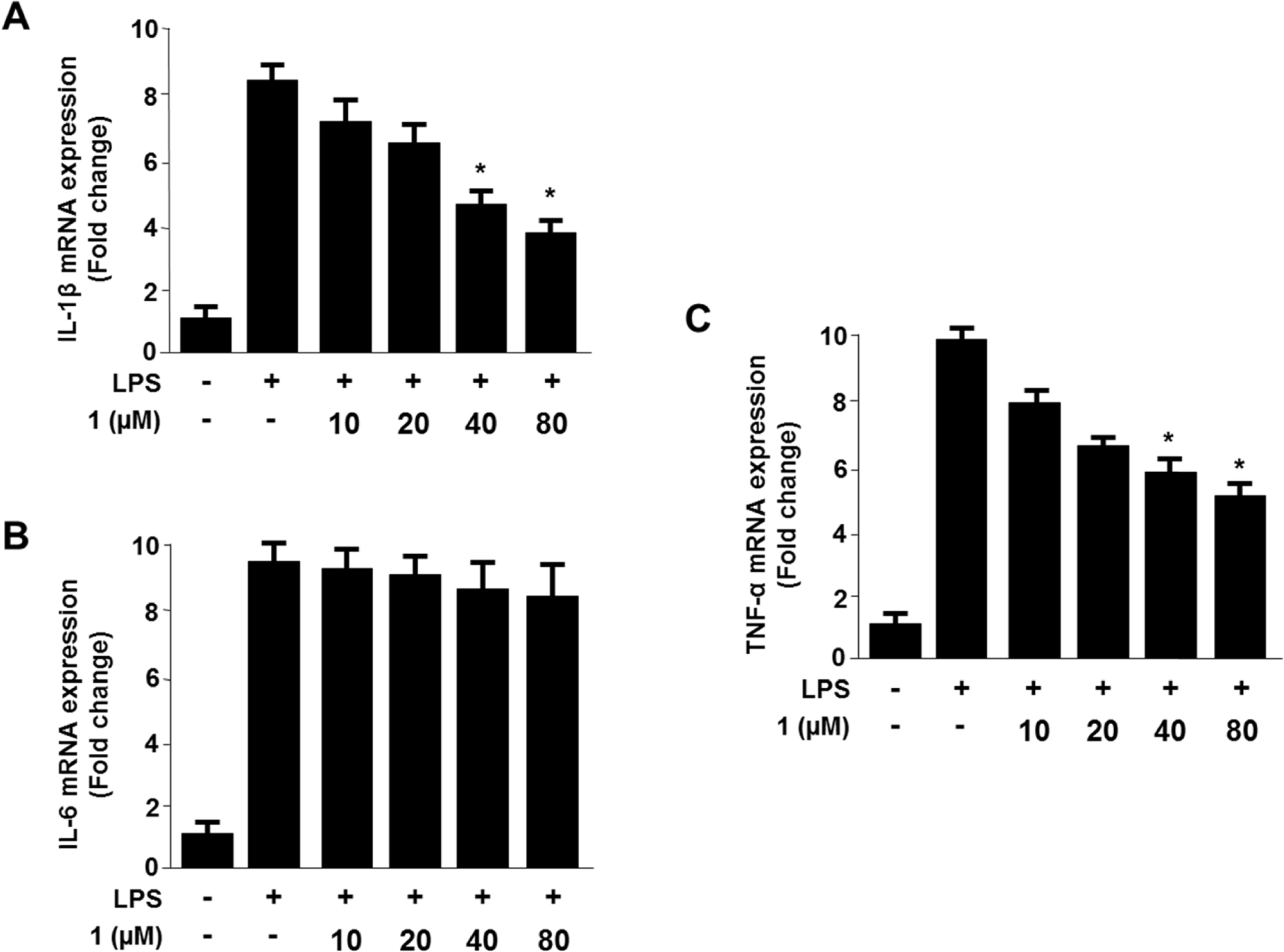
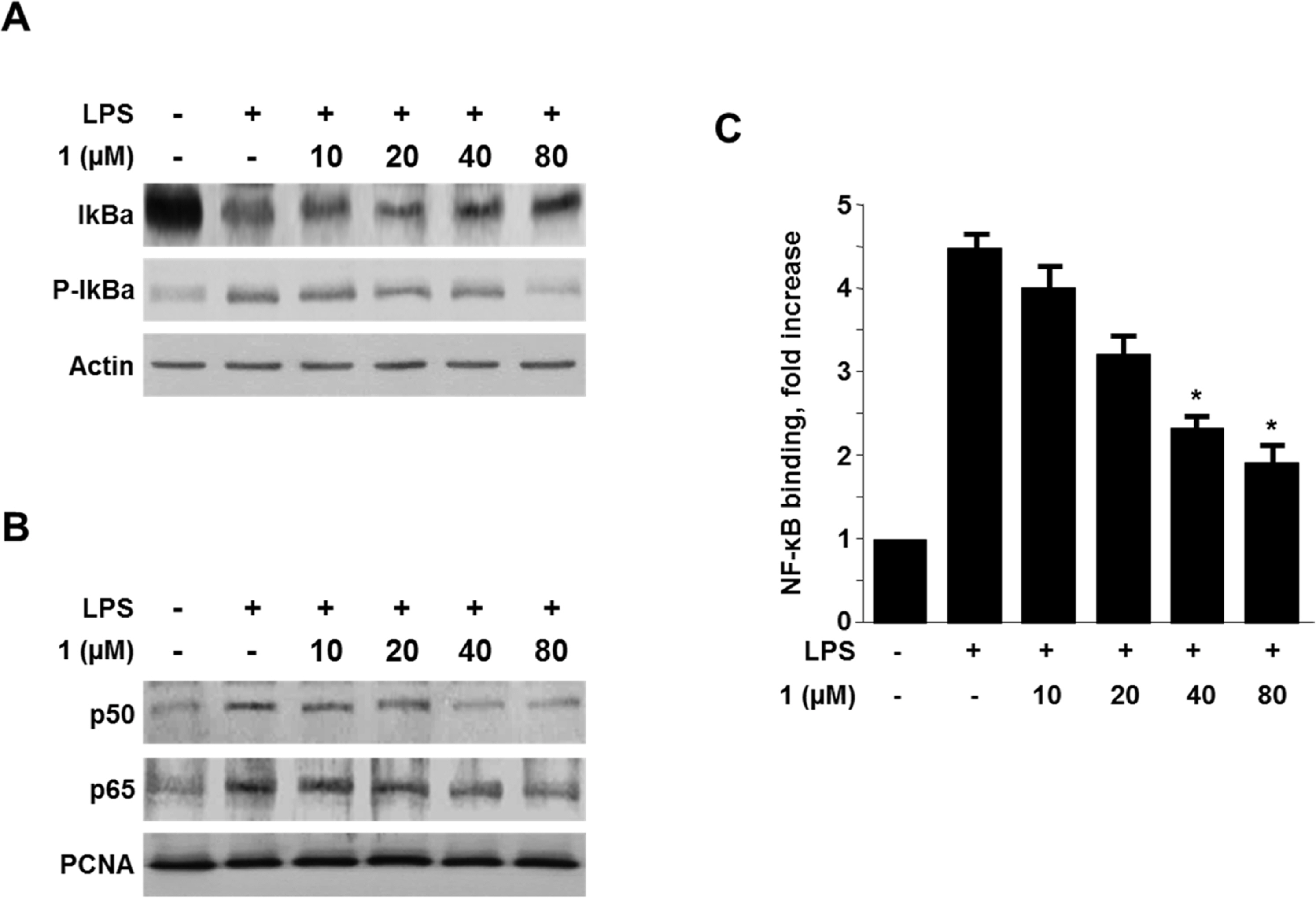
- Viridicatol (1) has previously been isolated from the extract of the marine-derived fungus Penicillium sp. SF-5295. In the course of further biological evaluation of this quinolone alkaloid, anti-inflammatory effect of 1 in RAW264.7 and BV2 cells stimulated with lipopolysaccharide (LPS) was observed. In this study, our data indicated that 1 suppressed the expression of well-known pro-inflammatory mediators such as inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2, and consequently inhibited the production of iNOS-derived nitric oxide (NO) and COX-2-derived prostaglandin E2 (PGE2) in LPS stimulated RAW264.7 and BV2 cells. Compound 1 also reduced mRNA expression of pro-inflammatory cytokines such as interleukin-1beta (IL-1beta), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-alpha). In the further evaluation of the mechanisms of these anti-inflammatory effects, 1 was shown to inhibit nuclear factor-kappa B (NF-kappaB) pathway in LPS-stimulated RAW264.7 and BV2 cells. Compound 1 blocked the phosphorylation and degradation of inhibitor kappa B (IkappaB)-alpha in the cytoplasm, and suppressed the translocation of NF-kappaB p65 and p50 heterodimer in nucleus. In addition, viridicatol (1) attenuated the DNA-binding activity of NF-kappaB in LPS-stimulated RAW264.7 and BV2 cells.
Keyword
MeSH Terms
-
Cytokines
Cytoplasm
Dinoprostone
Fungi
Interleukin-1beta
Interleukin-6
NF-kappa B*
Nitric Oxide
Nitric Oxide Synthase Type II
Penicillium*
Phosphorylation
Prostaglandin-Endoperoxide Synthases
RNA, Messenger
Tumor Necrosis Factor-alpha
Cytokines
Dinoprostone
Interleukin-1beta
Interleukin-6
NF-kappa B
Nitric Oxide
Nitric Oxide Synthase Type II
Prostaglandin-Endoperoxide Synthases
RNA, Messenger
Tumor Necrosis Factor-alpha
Figure
Reference
-
(1). Ingersoll M. A., Platt A. M., Potteaux S., Randolph G. J.Trends Immunol. 2011; 32:470–477.(2). Sosroseno W., Barid I., Herminajeng E., Susilowati H.Oral Microbiol. Immunol. 2002; 17:72–78.(3). Block M. L., Zecca L., Hong J. S.Nat. Rev. Neurosci. 2007; 8:57–69.(4). Jiang F., Ramanathan A., Miller M. T., Tang G. Q., Gale M. Jr., Patel S. S., Marcotrigiano J.Nature. 2011; 479:423–427.(5). Tsan M. F., Gao B. J.Endotoxin Res. 2007; 13:6–14.(6). Yang Y. Z., Tang Y. Z., Liu Y. H. J.Ethnopharmacol. 2013; 148:271–276.(7). Mizuno T., Kurotani T., Komatsu Y., Kawanokuchi J., Kato H., Mitsuma N., Suzumura A.Neuropharmacology. 2004; 46:404–411.(8). Ivashkiv L. B.Eur. J. Immunol. 2011; 41:2477–2481.(9). Baldwin A. S.Jr. Annu. Rev. Immunol. 1996; 14:649–683.
Article(10). Mancino A., Lawrence T.Clin. Cancer Res. 2010; 16:784–789.(11). Bremner P., Heinrich M. J.Pharm. Pharmacol. 2002; 54:453–472.(12). Rateb M. E., Ebel R.Nat. Prod. Rep. 2011; 28:290–334.(13). Bugni T. S., Ireland C. M.Nat. Prod. Rep. 2004; 21:143–163.(14). Fremlin L. J., Piggott A. M., Lacey E., Capon R. J. J.Nat. Prod. 2009; 72:666–670.(15). Sohn J. H., Lee Y. R., Lee D. S., Kim Y. C., Oh H. J.Microbiol. Biotechnol. 2013; 23:1206–1211.(16). Berridge M. V., Tan A. S.Arch. Biochem. Biophys. 1993; 303:474–482.(17). Titheradge M. A.Methods Mol. Biol. 1998; 100:83–91.(18). Karin M., Ben-Neriah Y.Annu. Rev. Immunol. 2000; 18:621–663.(19). Grilli M., Chiu J. J., Lenardo M. J.Int. Rev. Cytol. 1993; 143:1–62.(20). Chen F., Sun S. C., Kuh D. C., Gaydos L. J., Demers L. M.Biochem. Biophys. Res. Commun. 1995; 214:985–992.(21). Saleem M., Ali M. S., Hussain S., Jabbar A., Ashraf M., Lee Y. S.Nat. Prod. Rep. 2007; 24:1142–1152.(22). Fenical W., Jensen P. R.Nat. Chem. Biol. 2006; 2:666–673.(23). Lee D. S., Ko W., Quang T. H., Kim K. S., Sohn J. H., Jang J. H., Ahn J. S., Kim Y. C., Oh H.Mar. Drugs. 2013; 11:4510–4526.(24). Su X., Chen Q., Chen W., Chen T., Li W., Li Y., Dou X., Zhang Y., Shen Y., Wu H., Yu C.Int. Immunopharmacol. 2014; 19:88–93.(25). Wei M. Y., Yang R. Y., Shao C. L., Wang C. Y., Deng D. S., She Z. G., Lin Y. C.Chem. Nat. Compd. 2011; 47:322–325.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Shikonin Isolated from Lithospermum erythrorhizon Downregulates Proinflammatory Mediators in Lipopolysaccharide-Stimulated BV2 Microglial Cells by Suppressing Crosstalk between Reactive Oxygen Species and NF-kappaB
- Methyl p-Hydroxycinnamate Suppresses Lipopolysaccharide-Induced Inflammatory Responses through Akt Phosphorylation in RAW264.7 Cells
- Aromadendrin Inhibits Lipopolysaccharide-Induced Nuclear Translocation of NF-kappaB and Phosphorylation of JNK in RAW 264.7 Macrophage Cells
- Phosphorylation of Akt Mediates Anti-Inflammatory Activity of 1-p-Coumaroyl beta-D-Glucoside Against Lipopolysaccharide-Induced Inflammation in RAW264.7 Cells
- Anti-inflammatory effects of rutin in lipopolysaccharide-stimulated canine macrophage cells