Korean J Physiol Pharmacol.
2015 Sep;19(5):441-449. 10.4196/kjpp.2015.19.5.441.
Scutellarein Reduces Inflammatory Responses by Inhibiting Src Kinase Activity
- Affiliations
-
- 1Department of Genetic Engineering, Sungkyunkwan University, Suwon 440-746, Korea. jaecho@skku.edu
- 2School of Systems Biological Science, Soongsil University, Seoul 156-743, Korea. kimmy@ssu.ac.kr
- KMID: 2285589
- DOI: http://doi.org/10.4196/kjpp.2015.19.5.441
Abstract
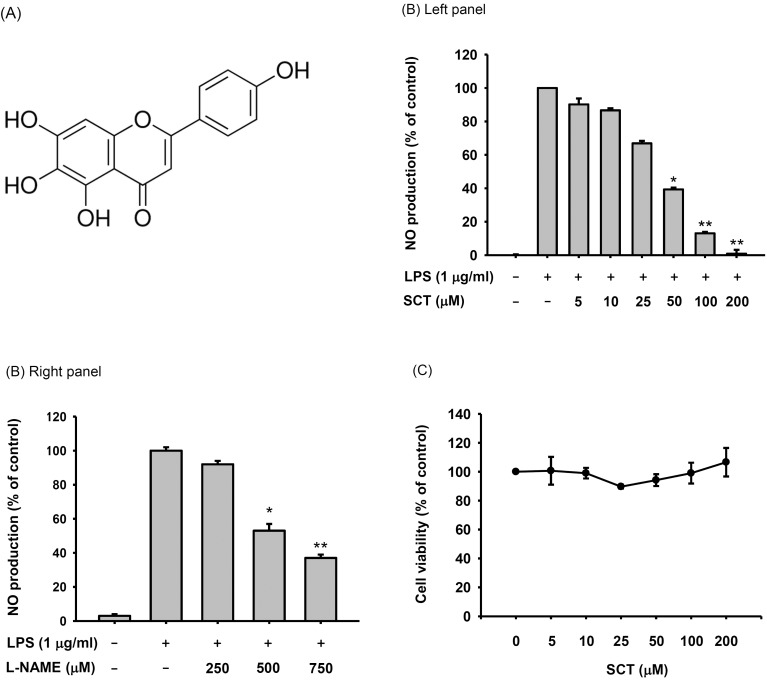
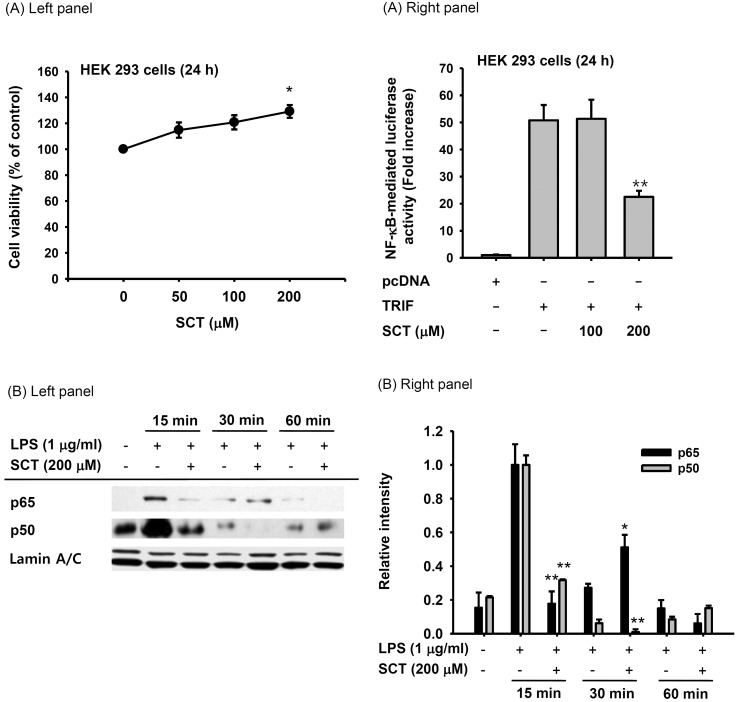
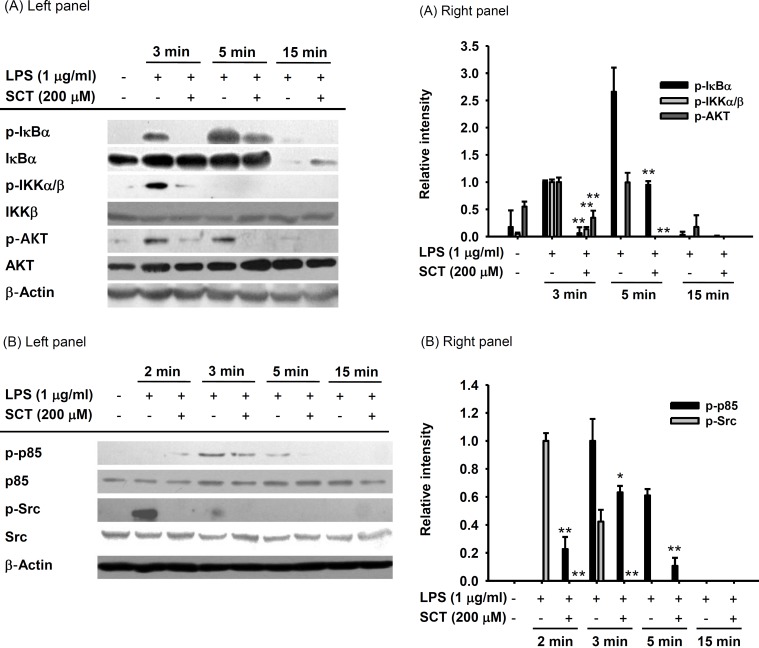
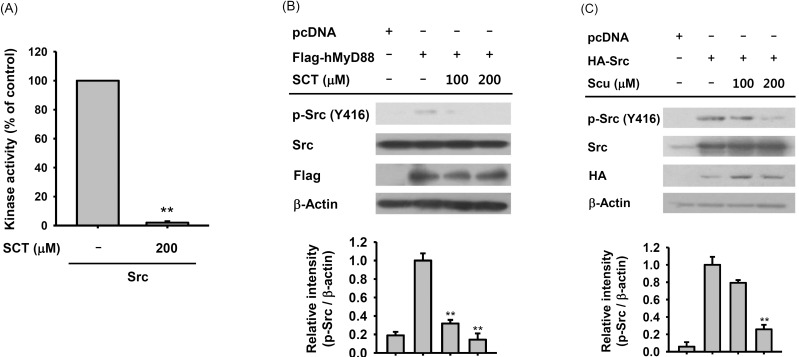
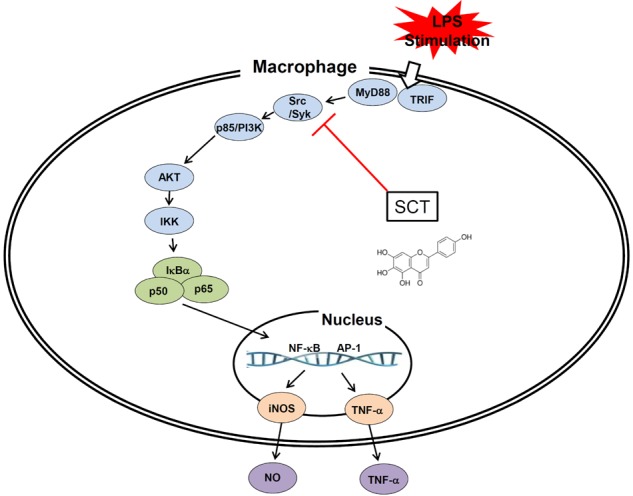
- Flavonoids are plant pigments that have been demonstrated to exert various pharmacological effects including anti-cancer, anti-diabetic, anti-atherosclerotic, anti-bacterial, and anti-inflammatory activities. However, the molecular mechanisms in terms of exact target proteins of flavonoids are not fully elucidated yet. In this study, we aimed to evaluate the anti-inflammatory mechanism of scutellarein (SCT), a flavonoid isolated from Erigeron breviscapus, Clerodendrum phlomidis and Oroxylum indicum Vent that have been traditionally used to treat various inflammatory diseases in China and Brazil. For this purpose, a nitric oxide (NO) assay, polymerase chain reaction (PCR), nuclear fractionation, immunoblot analysis, a kinase assay, and an overexpression strategy were employed. Scutellarein significantly inhibited NO production in a dose-dependent manner and reduced the mRNA expression levels of inducible NO synthase (iNOS) and tumor necrosis factor (TNF)-alpha in lipopolysaccharide (LPS)-activated RAW264.7 cells. In addition, SCT also dampened nuclear factor (NF)-kappaB-driven expression of a luciferase reporter gene upon transfection of a TIR-domain-containing adapter-inducing interferon-beta (TRIF) construct into Human embryonic kidney 293 (HEK 293) cells; similarly, NF-kappa B nuclear translocation was inhibited by SCT. Moreover, the phosphorylation levels of various upstream signaling enzymes involved in NF-kappaB activation were decreased by SCT treatment in LPS-treated RAW264.7 cells. Finally, SCT strongly inhibited Src kinase activity and also inhibited the autophosphorylation of overexpressed Src. Therefore, our data suggest that SCT can block the inflammatory response by directly inhibiting Src kinase activity linked to NF-kappaB activation.
Keyword
MeSH Terms
-
Brazil
China
Clerodendrum
Erigeron
Flavonoids
Genes, Reporter
Humans
Interferon-beta
Kidney
Luciferases
Macrophages
NF-kappa B
Nitric Oxide
Nitric Oxide Synthase
Phosphorylation
Phosphotransferases*
Plants
Polymerase Chain Reaction
RNA, Messenger
Transfection
Tumor Necrosis Factor-alpha
Flavonoids
Interferon-beta
Luciferases
NF-kappa B
Nitric Oxide
Nitric Oxide Synthase
Phosphotransferases
RNA, Messenger
Tumor Necrosis Factor-alpha
Figure
Cited by 2 articles
-
(
E )-3-(3-methoxyphenyl)-1-(2-pyrrolyl)-2-propenone displays suppression of inflammatory responses via inhibition of Src, Syk, and NF-κB
Yong Kim, Eun Jeong Jeong, In-Sook Han Lee, Mi-Yeon Kim, Jae Youl Cho
Korean J Physiol Pharmacol. 2016;20(1):91-99. doi: 10.4196/kjpp.2016.20.1.91.JS-III-49, a hydroquinone derivative, exerts anti-inflammatory activity by targeting Akt and p38
Young-Su Yi, Mi-Yeon Kim, Jae Youl Cho
Korean J Physiol Pharmacol. 2017;21(3):345-352. doi: 10.4196/kjpp.2017.21.3.345.
Reference
-
1. Kinne RW, Bräuer R, Stuhlmüller B, Palombo-Kinne E, Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Res. 2000; 2:189–202. PMID: 11094428.2. Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999; 340:115–126. PMID: 9887164.3. Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006; 290:L622–L645. PMID: 16531564.4. Stuhlmüller B, Ungethüm U, Scholze S, Martinez L, Backhaus M, Kraetsch HG, Kinne RW, Burmester GR. Identification of known and novel genes in activated monocytes from patients with rheumatoid arthritis. Arthritis Rheum. 2000; 43:775–790. PMID: 10765922.
Article5. Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005; 4:281–286. PMID: 16101534.
Article6. Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005; 17:1–14. PMID: 15585605.7. Doyle SL, O'Neill LA. Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol. 2006; 72:1102–1113. PMID: 16930560.8. Bresnihan B. Pathogenesis of joint damage in rheumatoid arthritis. J Rheumatol. 1999; 26:717–719. PMID: 10090189.9. Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013; 25:1939–1948. PMID: 23770291.
Article10. Mohan Maruga Raja MK, Mishra SH. Comprehensive review of Clerodendrum phlomidis: a traditionally used bitter. Zhong Xi Yi Jie He Xue Bao. 2010; 8:510–524. PMID: 20550872.
Article11. Harminder , Singh V, Chaudhary AK. A review on the taxonomy, ethnobotany, chemistry and pharmacology of Oroxylum indicum vent. Indian J Pharm Sci. 2011; 73:483–490. PMID: 22923859.12. Kim E, Yoon KD, Lee WS, Yang WS, Kim SH, Sung NY, Baek KS, Kim Y, Htwe KM, Kim YD, Hong S, Kim JH, Cho JY. Syk/Src-targeted anti-inflammatory activity of Codariocalyx motorius ethanolic extract. J Ethnopharmacol. 2014; 155:185–193. PMID: 24866386.
Article13. Tang H, Tang Y, Li N, Shi Q, Guo J, Shang E, Duan JA. Neuroprotective effects of scutellarin and scutellarein on repeatedly cerebral ischemia-reperfusion in rats. Pharmacol Biochem Behav. 2014; 118:51–59. PMID: 24423938.
Article14. Siraichi JT, Felipe DF, Brambilla LZ, Gatto MJ, Terra VA, Cecchini AL, Cortez LE, Rodrigues-Filho E, Cortez DA. Antioxidant capacity of the leaf extract obtained from Arrabidaea chica cultivated in Southern Brazil. PLoS One. 2013; 8:e72733. PMID: 24009700.
Article15. Yang KJ, Shin S, Piao L, Shin E, Li Y, Park KA, Byun HS, Won M, Hong J, Kweon GR, Hur GM, Seok JH, Chun T, Brazil DP, Hemmings BA, Park J. Regulation of 3-phosphoinositidedependent protein kinase-1 (PDK1) by Src involves tyrosine phosphorylation of PDK1 and Src homology 2 domain binding. J Biol Chem. 2008; 283:1480–1491. PMID: 18024423.
Article16. Byeon SE, Yu T, Yang Y, Lee YG, Kim JH, Oh J, Jeong HY, Hong S, Yoo BC, Cho WJ, Hong S, Cho JY. Hydroquinone regulates hemeoxygenase-1 expression via modulation of Src kinase activity through thiolation of cysteine residues. Free Radic Biol Med. 2013; 57:105–118. PMID: 23290930.
Article17. Shen T, Yang WS, Yi YS, Sung GH, Rhee MH, Poo H, Kim MY, Kim KW, Kim JH, Cho JY. AP-1/IRF-3 targeted anti-inflammatory activity of andrographolide isolated from Andrographis paniculata. Evid Based Complement Alternat Med. 2013; 2013:210736. PMID: 23840248.18. Kim DH, Chung JH, Yoon JS, Ha YM, Bae S, Lee EK, Jung KJ, Kim MS, Kim YJ, Kim MK, Chung HY. Ginsenoside Rd inhibits the expressions of iNOS and COX-2 by suppressing NF-κB in LPS-stimulated RAW264.7 cells and mouse liver. J Ginseng Res. 2013; 37:54–63. PMID: 23717157.
Article19. Youn CK, Park SJ, Lee MY, Cha MJ, Kim OH, You HJ, Chang IY, Yoon SP, Jeon YJ. Silibinin inhibits LPS-induced macrophage activation by blocking p38 MAPK in RAW 264.7 Cells. Biomol Ther (Seoul). 2013; 21:258–263. PMID: 24244809.
Article20. Lee YG, Chain BM, Cho JY. Distinct role of spleen tyrosine kinase in the early phosphorylation of inhibitor of kappaB alpha via activation of the phosphoinositide-3-kinase and Akt pathways. Int J Biochem Cell Biol. 2009; 41:811–821. PMID: 18775507.21. Zhang R, Zhu J, Cao HZ, Xie XL, Huang JJ, Chen XH, Luo ZY. Isolation and characterization of LHT-type plant amino acid transporter gene from Panax ginseng Meyer. J Ginseng Res. 2013; 37:361–370. PMID: 24198663.
Article22. Sohn SH, Kim SK, Kim YO, Kim HD, Shin YS, Yang SO, Kim SY, Lee SW. A comparison of antioxidant activity of Korean White and Red Ginsengs on H2O2-induced oxidative stress in HepG2 hepatoma cells. J Ginseng Res. 2013; 37:442–450. PMID: 24233437.
Article23. Byeon SE, Lee YG, Kim BH, Shen T, Lee SY, Park HJ, Park SC, Rhee MH, Cho JY. Surfactin blocks NO production in lipopolysaccharide-activated macrophages by inhibiting NFkappaB activation. J Microbiol Biotechnol. 2008; 18:1984–1989. PMID: 19131703.24. Lee JA, Lee MY, Shin IS, Seo CS, Ha H, Shin HK. Anti-inflammatory effects of Amomum compactum on RAW 264.7 cells via induction of heme oxygenase-1. Arch Pharm Res. 2012; 35:739–746. PMID: 22553068.25. Shen T, Lee J, Park MH, Lee YG, Rho HS, Kwak YS, Rhee MH, Park YC, Cho JY. Ginsenoside Rp1, a ginsenoside derivative, blocks promoter activation of iNOS and COX-2 genes by suppression of an IKKβ-mediated NF-κB pathway in HEK293 cells. J Ginseng Res. 2011; 35:200–208. PMID: 23717062.
Article26. Song SB, Tung NH, Quang TH, Ngan NT, Kim KE, Kim YH. Inhibition of TNF-α-mediated NF-κB transcriptional activity in HepG2 cells by Dammarane-type Saponins from Panax ginseng leaves. J Ginseng Res. 2012; 36:146–152. PMID: 23717114.
Article27. Jung KK, Lee HS, Cho JY, Shin WC, Rhee MH, Kim TG, Kang JH, Kim SH, Hong S, Kang SY. Inhibitory effect of curcumin on nitric oxide production from lipopolysaccharide-activated primary microglia. Life Sci. 2006; 79:2022–2031. PMID: 16934299.
Article28. Tan ZH, Yu LH, Wei HL, Liu GT. The protective action of scutellarin against immunological liver injury induced by concanavalin A and its effect on pro-inflammatory cytokines in mice. J Pharm Pharmacol. 2007; 59:115–121. PMID: 17227628.
Article29. Rao YK, Fang SH, Hsieh SC, Yeh TH, Tzeng YM. The constituents of Anisomeles indica and their anti-inflammatory activities. J Ethnopharmacol. 2009; 121:292–296. PMID: 19041702.
Article30. Pandith H, Zhang X, Thongpraditchote S, Wongkrajang Y, Gritsanapan W, Baek SJ. Effect of Siam weed extract and its bioactive component scutellarein tetramethyl ether on anti-inflammatory activity through NF-κB pathway. J Ethnopharmacol. 2013; 147:434–441. PMID: 23535395.
Article31. Clancy RM, Abramson SB. Nitric oxide: a novel mediator of inflammation. Proc Soc Exp Biol Med. 1995; 210:93–101. PMID: 7568290.
Article32. Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001; 2:907–916. PMID: 11577346.
Article33. Cheng CY, Hu CC, Yang HJ, Lee MC, Kao ES. Inhibitory effects of scutellarein on proliferation of human lung cancer A549 cells through ERK and NFκB mediated by the EGFR pathway. Chin J Physiol. 2014; 57:182–187. PMID: 25246059.
Article34. Kim MH, Yoo DS, Lee SY, Byeon SE, Lee YG, Min T, Rho HS, Rhee MH, Lee J, Cho JY. The TRIF/TBK1/IRF-3 activation pathway is the primary inhibitory target of resveratrol, contributing to its broad-spectrum anti-inflammatory effects. Pharmazie. 2011; 66:293–300. PMID: 21612158.35. O'Shea JM, Perkins ND. Regulation of the RelA (p65) transactivation domain. Biochem Soc Trans. 2008; 36:603–608. PMID: 18631125.36. Endale M, Park SC, Kim S, Kim SH, Yang Y, Cho JY, Rhee MH. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology. 2013; 218:1452–1467. PMID: 23735482.
Article37. Chen KC, Chen CY, Lin CR, Yang TY, Chen TH, Wu LC, Wu CC. Luteolin attenuates TGF-β1-induced epithelial-mesenchymal transition of lung cancer cells by interfering in the PI3K/Akt-NF-κB-Snail pathway. Life Sci. 2013; 93:924–933. PMID: 24140887.
Article38. Chen X, Yang X, Liu T, Guan M, Feng X, Dong W, Chu X, Liu J, Tian X, Ci X, Li H, Wei J, Deng Y, Deng X, Chi G, Sun Z. Kaempferol regulates MAPKs and NF-κB signaling pathways to attenuate LPS-induced acute lung injury in mice. Int Immunopharmacol. 2012; 14:209–216. PMID: 22835426.
Article39. Eo SH, Cho H, Kim SJ. Resveratrol inhibits nitric oxideinduced apoptosis via the NF-Kappa B pathway in rabbit articular chondrocytes. Biomol Ther (Seoul). 2013; 21:364–370. PMID: 24244824.
Article40. Batra S, Balamayooran G, Sahoo MK. Nuclear factor-κB: a key regulator in health and disease of lungs. Arch Immunol Ther Exp (Warsz). 2011; 59:335–351. PMID: 21786215.
Article41. Byeon SE, Yi YS, Oh J, Yoo BC, Hong S, Cho JY. The role of Src kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2012; 2012:512926. PMID: 23209344.
Article42. Lee YG, Lee J, Byeon SE, Yoo DS, Kim MH, Lee SY, Cho JY. Functional role of Akt in macrophage-mediated innate immunity. Front Biosci (Landmark Ed). 2011; 16:517–530. PMID: 21196185.
Article43. Huang CH, Mandelker D, Gabelli SB, Amzel LM. Insights into the oncogenic effects of PIK3CA mutations from the structure of p110alpha/p85alpha. Cell Cycle. 2008; 7:1151–1156. PMID: 18418043.
Article44. Osusky M, Taylor SJ, Shalloway D. Autophosphorylation of purified c-Src at its primary negative regulation site. J Biol Chem. 1995; 270:25729–25732. PMID: 7592753.
Article45. Yang Y, Yu T, Lee YG, Yang WS, Oh J, Jeong D, Lee S, Kim TW, Park YC, Sung GH, Cho JY. Methanol extract of Hopea odorata suppresses inflammatory responses via the direct inhibition of multiple kinases. J Ethnopharmacol. 2013; 145:598–607. PMID: 23220195.46. Jeong D, Yang WS, Yang Y, Nam G, Kim JH, Yoon DH, Noh HJ, Lee S, Kim TW, Sung GH, Cho JY. In vitro and in vivo anti-inflammatory effect of Rhodomyrtus tomentosa methanol extract. J Ethnopharmacol. 2013; 146:205–213. PMID: 23295168.
Article47. Seo SG, Yang H, Shin SH, Min S, Kim YA, Yu JG, Lee DE, Chung MY, Heo YS, Kwon JY, Yue S, Kim KH, Cheng JX, Lee KW, Lee HJ. A metabolite of daidzein, 6,7,4'-trihydroxyisoflavone, suppresses adipogenesis in 3T3-L1 preadipocytes via ATP-competitive inhibition of PI3K. Mol Nutr Food Res. 2013; 57:1446–1455. PMID: 23737351.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Src Kinase Regulates Nitric Oxide-induced Dedifferentiation and Cyclooxygenase-2 Expression in Articular Chondrocytes via p38 Kinase-dependent Pathway
- Endoplasmic reticulum stress (ER-stress) by 2-deoxy-D-glucose (2DG) reduces cyclooxygenase-2 (COX-2) expression and N-glycosylation and induces a loss of COX-2 activity via a Src kinase-dependent pathway in rabbit articular chondrocytes
- Beauvericin, a cyclic peptide, inhibits inflammatory responses in macrophages by inhibiting the NF-κB pathway
- Involvement of Src Family Tyrosine Kinase in Apoptosis of Human Neutrophils Induced by Protozoan Parasite Entamoeba histolytica
- Antimetastatic effect of fucoidan against non-small cell lung cancer by suppressing non-receptor tyrosine kinase and extracellular signalrelated kinase pathway