Korean J Physiol Pharmacol.
2014 Aug;18(4):347-352. 10.4196/kjpp.2014.18.4.347.
Effects of Watercress Containing Rutin and Rutin Alone on the Proliferation and Osteogenic Differentiation of Human Osteoblast-like MG-63 Cells
- Affiliations
-
- 1College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea. hahyung@cau.ac.kr
- KMID: 2285530
- DOI: http://doi.org/10.4196/kjpp.2014.18.4.347
Abstract
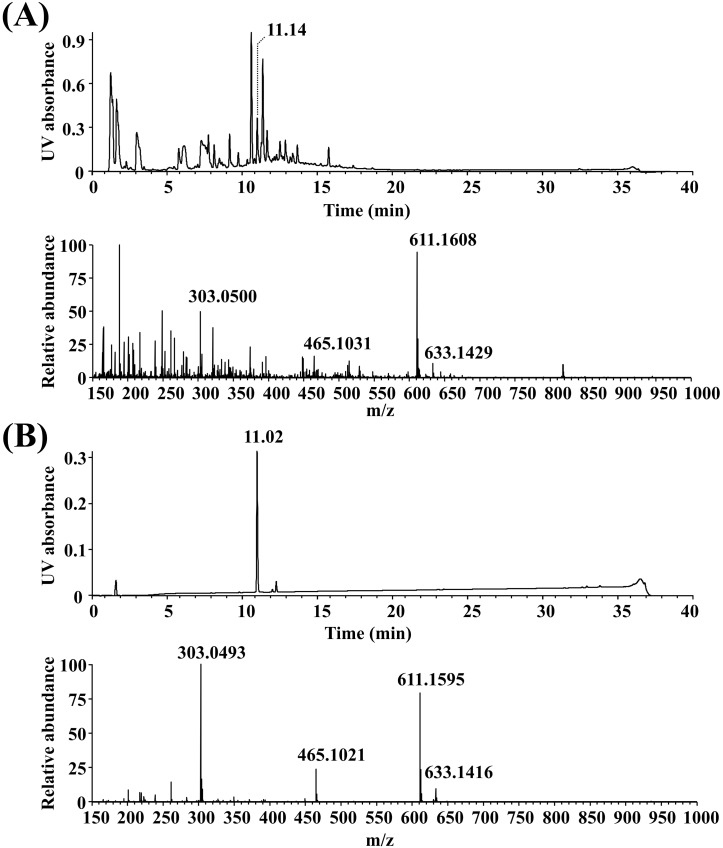
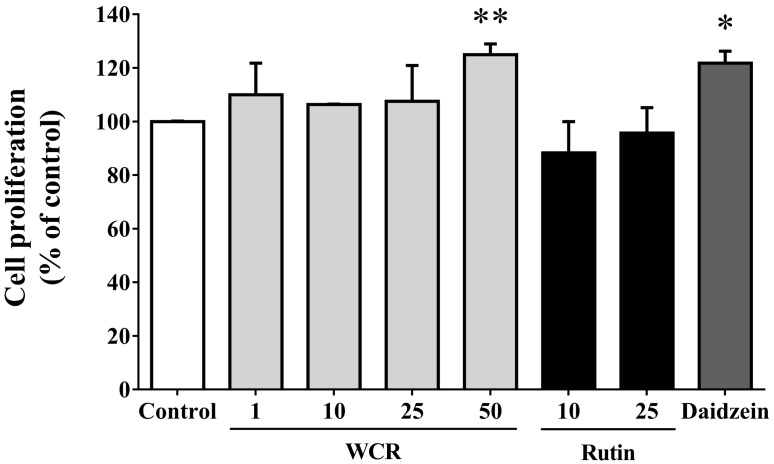
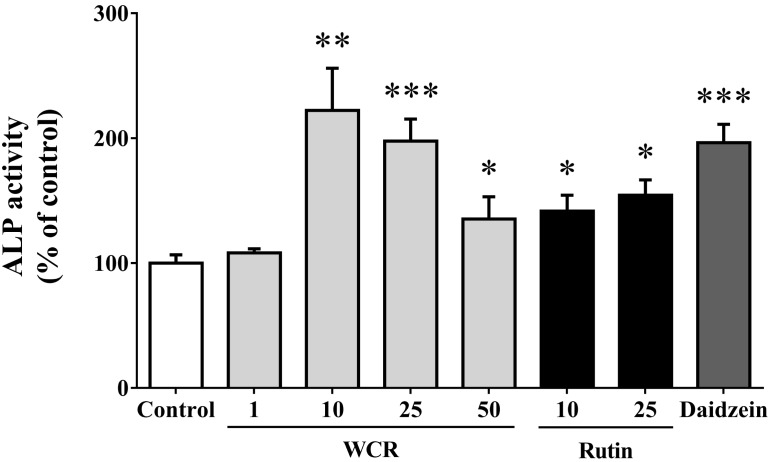
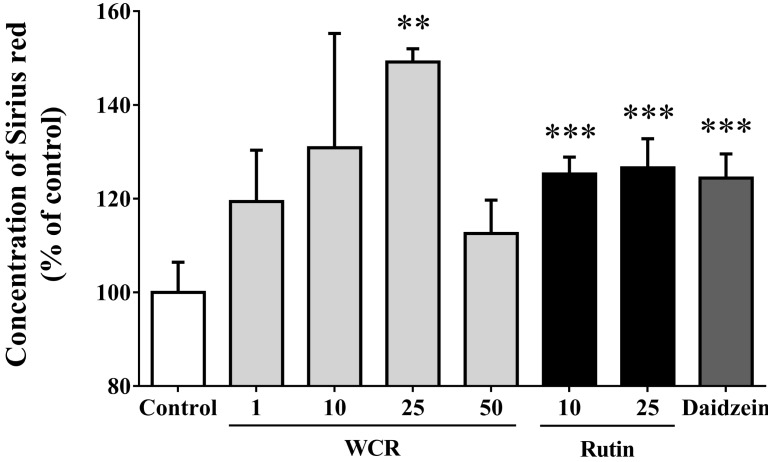
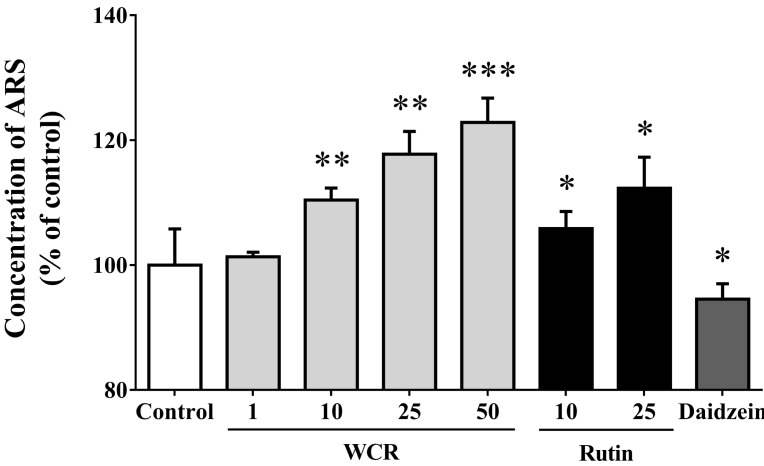
- Most known osteoporosis medicines are effective for bone resorption, and so there is an increasing demand for medicines that stimulate bone formation. Watercress (N. officinale R. Br.) is widely used as a salad green and herbal remedy. This study analyzed a watercress extract using ultra-performance liquid chromatography/mass spectrometry, and identified a rutin as one of its major constituents. Osteogenic-related assays were used to compare the effects of watercress containing rutin (WCR) and rutin alone on the proliferation and differentiation of human osteoblast-like MG-63 cells. The reported data are expressed as percentages relative to the control value (medium alone; assigned as 100%). WCR increased cell proliferation to 125.0+/-4.0% (mean+/-SD), as assessed using a cell viability assay, and increased the activity of alkaline phosphatase, an early differentiation marker, to 222.3+/-33.8%. In addition, WCR increased the expression of collagen type I, another early differentiation marker, to 149.2+/-2.8%, and increased the degree of mineralization, a marker of the late process of differentiation, to 122.9+/-3.9%. Rutin alone also increased the activity of ALP (to 154.4+/-12.2%), the expression of collagen type I (to 126.6+/-6.2%), and the degree of mineralization (to 112.3+/-5.0%). Daidzein, which is reported to stimulate bone formation, was used as a positive control; the effects of WCR on proliferation and differentiation were significantly greater than those of daidzein. These results indicate that WCR and rutin can both induce bone formation via the differentiation of MG-63 cells. This is the first study demonstrating the effectiveness of either WCR or rutin as an osteoblast stimulant.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Effects of Free Anthraquinones Extract from the Rhubarb on Cell Proliferation and Accumulation of Extracellular Matrix in High Glucose Cultured-Mesangial Cells
Jianyun Wang, Hui Fang, Bingzheng Dong, Dongdong Wang, Yan Li, Xiao Chen, Lijuan Chen, Tong Wei, Qunli Wei
Korean J Physiol Pharmacol. 2015;19(6):485-489. doi: 10.4196/kjpp.2015.19.6.485.
Reference
-
1. Bhutani G, Gupta MC. Emerging therapies for the treatment of osteoporosis. J Midlife Health. 2013; 4:147–152. PMID: 24672186.
Article2. Lau RY, Guo X. A Review on current osteoporosis research: with special focus on disuse bone loss. J Osteoporos. 2011; 2011:293808. PMID: 21876833.
Article3. Roux S. New treatment targets in osteoporosis. Joint Bone Spine. 2010; 77:222–228. PMID: 20381400.
Article4. Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, Kendler DL, McClung MR, Miller PD, Olszynski WP, Orwoll E, Yuen CK. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005; 26:688–703. PMID: 15769903.
Article5. Bakhsh A, Mustapha NM, Mohamed S. Catechin-rich oil palm leaf extract enhances bone calcium content of estrogen-deficient rats. Nutrition. 2013; 29:667–672. PMID: 23290096.
Article6. Gonçalves EM, Cruz RMS, Abreu M, Brandão TRS, Silva CLM. Biochemical and colour changes of watercress (Nasturtium officinale R. Br.) during freezing and frozen storage. J Food Eng. 2009; 93:32–39.7. Zahradníková H, Petříková K. Nematocid effects of watercress (Nasturtium officinale R. Br.). Acta Univ Agric Silvic Mendelianae Brun. 2013; 61:233–236.8. Amiri H. Volatile constituents and antioxidant activity of flowers, stems and leaves of Nasturtium officinale R. Br. Nat Prod Res. 2012; 26:109–115. PMID: 21815727.9. Ozen T. Investigation of antioxidant properties of Nasturtium officinale (watercress) leaf extracts. Acta Pol Pharm. 2009; 66:187–193. PMID: 19719054.10. Manach C, Morand C, Crespy V, Demigné C, Texier O, Régérat F, Rémésy C. Quercetin is recovered in human plasma as conjugated derivatives which retain antioxidant properties. FEBS Lett. 1998; 426:331–336. PMID: 9600261.
Article11. Tamura G, Gold C, Ferro-Luzzi A, Ames BN. Fecalase: a model for activation of dietary glycosides to mutagens by intestinal flora. Proc Natl Acad Sci U S A. 1980; 77:4961–4965. PMID: 6933540.
Article12. Prouillet C, Mazière JC, Mazière C, Wattel A, Brazier M, Kamel S. Stimulatory effect of naturally occurring flavonols quercetin and kaempferol on alkaline phosphatase activity in MG-63 human osteoblasts through ERK and estrogen receptor pathway. Biochem Pharmacol. 2004; 67:1307–1313. PMID: 15013846.
Article13. Notoya M, Tsukamoto Y, Nishimura H, Woo JT, Nagai K, Lee IS, Hagiwara H. Quercetin, a flavonoid, inhibits the proliferation, differentiation, and mineralization of osteoblasts in vitro. Eur J Pharmacol. 2004; 485:89–96. PMID: 14757127.
Article14. La Casa C, Villegas I, Alarcón de la Lastra C, Motilva V, Martín Calero MJ. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J Ethnopharmacol. 2000; 71:45–53. PMID: 10904145.
Article15. Horcajada-Molteni MN, Crespy V, Coxam V, Davicco MJ, Rémésy C, Barlet JP. Rutin inhibits ovariectomy-induced osteopenia in rats. J Bone Miner Res. 2000; 15:2251–2258. PMID: 11092407.
Article16. Srivastava S, Bankar R, Roy P. Assessment of the role of flavonoids for inducing osteoblast differentiation in isolated mouse bone marrow derived mesenchymal stem cells. Phytomedicine. 2013; 20:683–690. PMID: 23570998.
Article17. Tominaga H, Ishiyama M, Ohseto F, Sasamoto K, Hamamoto T, Suzuki K, Watanabe M. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal Commun. 1999; 36:47–50.
Article18. Rivkin RB, Swift E. Characterization of alkaline phosphatase and organic phosphorous utilization in the oceanic dinoflagellate Pyrocystis noctiluca. Mar Biol. 1980; 61:1–8.19. Tullberg-Reinert H, Jundt G. In situ measurement of collagen synthesis by human bone cells with a sirius red-based colorimetric microassay: effects of transforming growth factor beta2 and ascorbic acid 2-phosphate. Histochem Cell Biol. 1999; 112:271–276. PMID: 10550611.20. Boyd LA, McCann MJ, Hashim Y, Bennett RN, Gill CI, Rowland IR. Assessment of the anti-genotoxic, anti-proliferative, and anti-metastatic potential of crude watercress extract in human colon cancer cells. Nutr Cancer. 2006; 55:232–241. PMID: 17044779.
Article21. Spence RMM, Tucknott OG, Baker EA, Holloway PJ. Compounds associated with the surface lipid layer of watercress. Phytochemistry. 1983; 22:1753–1756.
Article22. Park NI, Kim JK, Park WT, Cho JW, Lim YP, Park SU. An efficient protocol for genetic transformation of watercress (Nasturtium officinale) using Agrobacterium rhizogenes. Mol Biol Rep. 2011; 38:4947–4953. PMID: 21161399.23. Martínez-Sánchez A, Gil-Izquierdo A, Gil MI, Ferreres F. A comparative study of flavonoid compounds, vitamin C, and antioxidant properties of baby leaf Brassicaceae species. J Agric Food Chem. 2008; 56:2330–2340. PMID: 18321050.
Article24. Lee HS, Jung EY, Bae SH, Kwon KH, Kim JM, Suh HJ. Stimulation of osteoblastic differentiation and mineralization in MC3T3-E1 cells by yeast hydrolysate. Phytother Res. 2011; 25:716–723. PMID: 21077261.
Article25. Reffitt DM, Ogston N, Jugdaohsingh R, Cheung HF, Evans BA, Thompson RP, Powell JJ, Hampson GN. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone. 2003; 32:127–135. PMID: 12633784.
Article26. Blumenthal NC, Posner AS. Hydroxyapatite: mechanism of formation and properties. Calcif Tissue Res. 1973; 13:235–243. PMID: 4792086.
Article27. Xiong Y, Yang HJ, Feng J, Shi ZL, Wu LD. Effects of alendronate on the proliferation and osteogenic differentiation of MG-63 cells. J Int Med Res. 2009; 37:407–416. PMID: 19383235.
Article28. Ge Y, Chen D, Xie L, Zhang R. Enhancing effect of daidzein on the differentiation and mineralization in mouse osteoblast-like MC3T3-E1 cells. Yakugaku Zasshi. 2006; 126:651–656. PMID: 16880723.
Article29. Gao YH, Yamaguchi M. Anabolic effect of daidzein on cortical bone in tissue culture: comparison with genistein effect. Mol Cell Biochem. 1999; 194:93–98. PMID: 10391128.30. Sugimoto E, Yamaguchi M. Stimulatory effect of daidzein in osteoblastic MC3T3-E1 cells. Biochem Pharmacol. 2000; 59:471–475. PMID: 10660113.
Article31. Lee YS, Chen X, Anderson JJ. Physiological concentrations of genistein stimulate the proliferation and protect against free radical-induced oxidative damage of MC3T3-E1 osteoblast-like cells. Nutr Res. 2001; 21:1287–1298.
Article32. Kanno S, Hirano S, Kayama F. Effects of phytoestrogens and environmental estrogens on osteoblastic differentiation in MC3T3-E1 cells. Toxicology. 2004; 196:137–145. PMID: 15036763.
Article33. Michaelsen KF, Astrup AV, Mosekilde L, Richelsen B, Schroll M, Sørensen OH. The importance of nutrition for the prevention of osteoporosis. Ugeskr Laeger. 1994; 156:958–960. 963–965. PMID: 8009738.34. Zhang Y, Yu L, Ao M, Jin W. Effect of ethanol extract of Lepidium meyenii Walp. on osteoporosis in ovariectomized rat. J Ethnopharmacol. 2006; 105:274–279. PMID: 16466876.35. Van Kiem P, Quang TH, Huong TT, Nhung le TH, Cuong NX, Van Minh C, Choi EM, Kim YH. Chemical constituents of Acanthus ilicifolius L. and effect on osteoblastic MC3T3E1 cells. Arch Pharm Res. 2008; 31:823–829. PMID: 18704321.36. Tai BH, Cuong NM, Huong TT, Choi EM, Kim JA, Kim YH. Chrysoeriol isolated from the leaves of Eurya ciliata stimulates proliferation and differentiation of osteoblastic MC3T3-E1 cells. J Asian Nat Prod Res. 2009; 11:817–823. PMID: 20183330.37. Park SH, Nhiem NX, Kiem PV, Choi EM, Kim JA, Kim YH. A new norlupane triterpene from the leaves of Acanthopanax koreanum increases the differentiation of osteoblastic MC3T3-e1 cells. Arch Pharm Res. 2010; 33:75–80. PMID: 20191347.38. Ha H, Ho J, Shin S, Kim H, Koo S, Kim IH, Kim C. Effects of Eucommiae cortex on osteoblast-like cell proliferation and osteoclast inhibition. Arch Pharm Res. 2003; 26:929–936. PMID: 14661859.
Article39. Shin JM, Park CK, Shin EJ, Jo TH, Hwang IK. Effects of Scutellaria radix extract on osteoblast differentiation and osteoclast formation. Korean J Food Sci Technol. 2008; 40:674–679.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Rutin on the Melanogenesis and Nitric Oxide in UVB-irradiated HM3KO Human Melanoma
- Rutin induces autophagy in cancer cells
- Influence of rutin on the effects of neonatal cigarette smoke exposure-induced exacerbated MMP-9 expression, Th17 cytokines and NF-κB/iNOS-mediated inflammatory responses in asthmatic mice model
- Identification and HPLC Quantification of a Phytoecdysone and Three Phenolic Glycosides in Lamium takesimense Nakai
- Rutin inhibits osteoclast formation by decreasing reactive oxygen species and TNF-alpha by inhibiting activation of NF-kappaB