Korean J Physiol Pharmacol.
2013 Aug;17(4):267-274. 10.4196/kjpp.2013.17.4.267.
Curcumin Attenuates Radiation-Induced Inflammation and Fibrosis in Rat Lungs
- Affiliations
-
- 1Department of Internal Medicine, Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju 660-290, Korea. ljd8611@empal.com
- 2Department of Anatomy, Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju 660-290, Korea. anaroh@gnu.ac.kr
- 3Department of Radiation Oncology, Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju 660-290, Korea.
- 4Department of Thoracic and Cardiovascular Surgery, Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju 660-290, Korea.
- KMID: 2285460
- DOI: http://doi.org/10.4196/kjpp.2013.17.4.267
Abstract
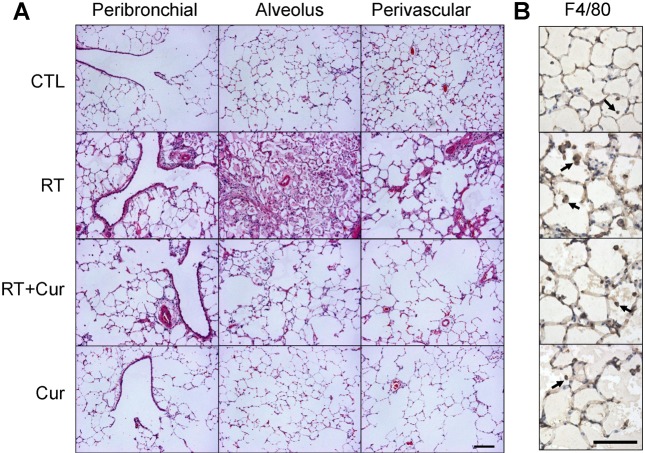
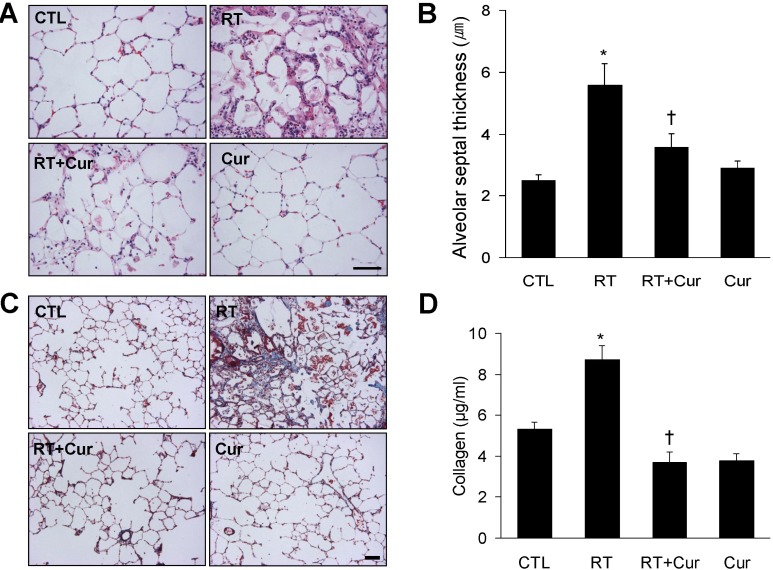
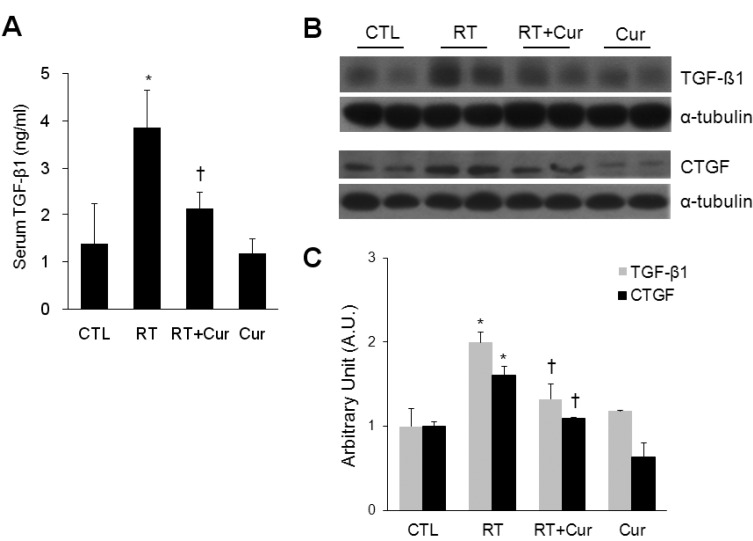
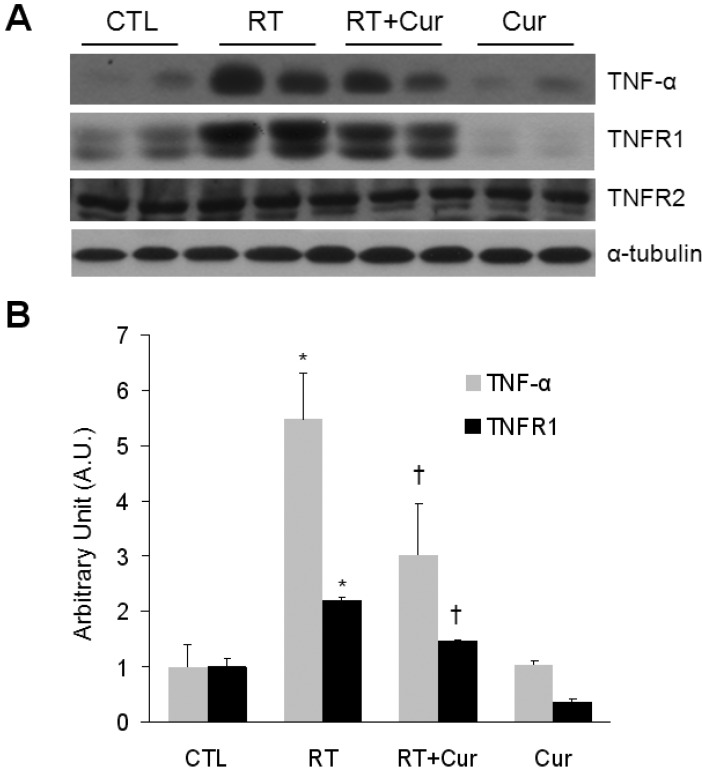
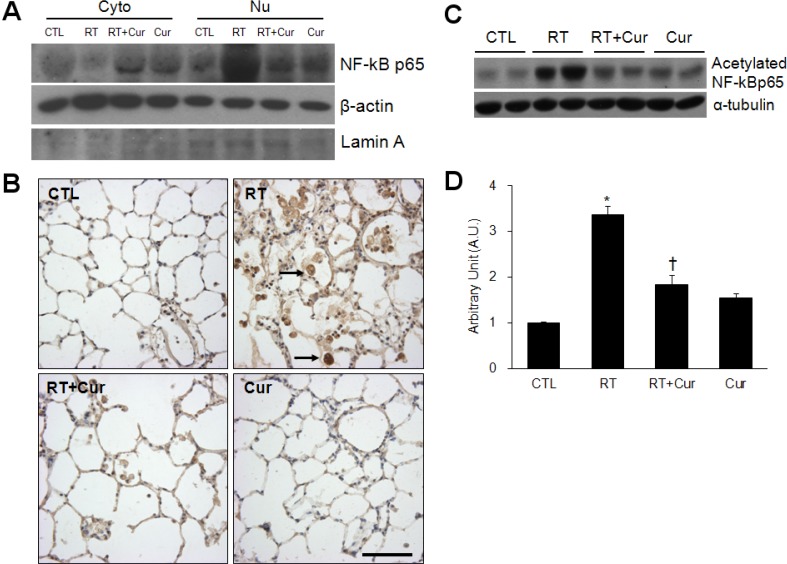
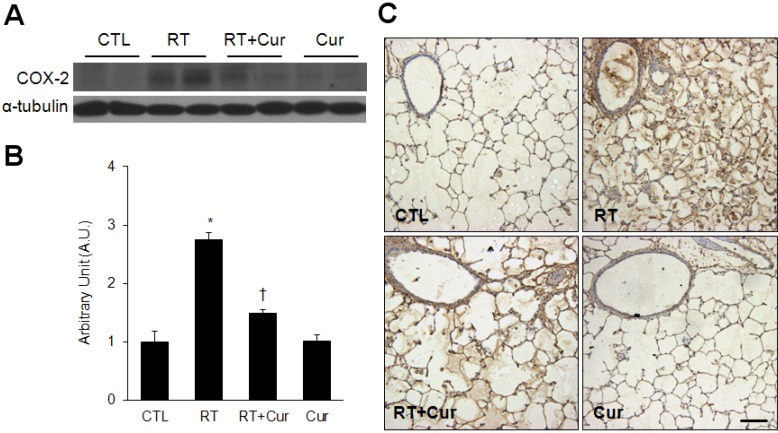
- A beneficial radioprotective agent has been used to treat the radiation-induced lung injury. This study was performed to investigate whether curcumin, which is known to have anti-inflammatory and antioxidant properties, could ameliorate radiation-induced pulmonary inflammation and fibrosis in irradiated lungs. Rats were given daily doses of intragastric curcumin (200 mg/kg) prior to a single irradiation and for 8 weeks after radiation. Histopathologic findings demonstrated that macrophage accumulation, interstitial edema, alveolar septal thickness, perivascular fibrosis, and collapse in radiation-treated lungs were inhibited by curcumin administration. Radiation-induced transforming growth factor-beta1 (TGF-beta1), connective tissue growth factor (CTGF) expression, and collagen accumulation were also inhibited by curcumin. Moreover, western blot analysis revealed that curcumin lowered radiation-induced increases of tumor necrosis factor-alpha (TNF-alpha), TNF receptor 1 (TNFR1), and cyclooxygenase-2 (COX-2). Curcumin also inhibited the nuclear translocation of nuclear factor-kappa B (NF-kappaB) p65 in radiation-treated lungs. These results indicate that long-term curcumin administration may reduce lung inflammation and fibrosis caused by radiation treatment.
Keyword
MeSH Terms
-
Animals
Blotting, Western
Collagen
Connective Tissue Growth Factor
Curcumin
Cyclooxygenase 2
Edema
Fibrosis
Inflammation
Lung
Lung Injury
Macrophages
Pneumonia
Rats
Receptors, Tumor Necrosis Factor
Tumor Necrosis Factor-alpha
Collagen
Connective Tissue Growth Factor
Curcumin
Cyclooxygenase 2
Receptors, Tumor Necrosis Factor
Tumor Necrosis Factor-alpha
Figure
Cited by 1 articles
-
Curcumin protects against the intestinal ischemia-reperfusion injury: involvement of the tight junction protein ZO-1 and TNF-α related mechanism
Shuying Tian, Ruixue Guo, Sichen Wei, Yu Kong, Xinliang Wei, Weiwei Wang, Xiaomeng Shi, Hongyu Jiang
Korean J Physiol Pharmacol. 2016;20(2):147-152. doi: 10.4196/kjpp.2016.20.2.147.
Reference
-
1. Rothwell RI, Kelly SA, Joslin CA. Radiation pneumonitis in patients treated for breast cancer. Radiother Oncol. 1985; 4:9–14. PMID: 3929337.
Article2. Tarbell NJ, Thompson L, Mauch P. Thoracic irradiation in Hodgkin's disease: disease control and long-term complications. Int J Radiat Oncol Biol Phys. 1990; 18:275–281. PMID: 2105920.
Article3. Maasilta P, Holsti LR, Blomqvist P, Kivisaari L, Mattson K. N-acetylcysteine in combination with radiotherapy in the treatment of non-small cell lung cancer: a feasibility study. Radiother Oncol. 1992; 25:192–195. PMID: 1335155.
Article4. Thresiamma KC, George J, Kuttan R. Protective effect of curcumin, ellagic acid and bixin on radiation induced toxicity. Indian J Exp Biol. 1996; 34:845–847. PMID: 9014516.5. Redlich CA, Rockwell S, Chung JS, Sikora AG, Kelley M, Mayne ST. Vitamin A inhibits radiation-induced pneumonitis in rats. J Nutr. 1998; 128:1661–1664. PMID: 9772133.
Article6. Antonadou D, Petridis A, Synodinou M, Throuvalas N, Bolanos N, Veslemes M, Sagriotis A. Amifostine reduces radiochemotherapy-induced toxicities in patients with locally advanced non-small cell lung cancer. Semin Oncol. 2003; 30(6 Suppl 18):2–9. PMID: 14727235.
Article7. Anscher MS, Kong FM, Jirtle RL. The relevanceof transforming growth factor beta 1 in pulmonary injury after radiation therapy. Lung Cancer. 1998; 19:109–120. PMID: 9567247.8. Anscher MS, Chen L, Rabbani Z, Kang S, Larrier N, Huang H, Samulski TV, Dewhirst MW, Brizel DM, Folz RJ, Vujaskovic Z. Recent progress in defining mechanisms and potential targets for prevention of normal tissue injury after radiation therapy. Int J Radiat Oncol Biol Phys. 2005; 62:255–259. PMID: 15850930.
Article9. O'Sullivan B, Levin W. Late radiation-related fibrosis: pathogenesis, manifestations, and current management. Semin Radiat Oncol. 2003; 13:274–289. PMID: 12903016.10. Anscher MS, Thrasher B, Rabbani Z, Teicher B, Vujaskovic Z. Antitransforming growth factor-beta antibody 1D11 ameliorates normal tissue damage caused by high-dose radiation. Int J Radiat Oncol Biol Phys. 2006; 65:876–881. PMID: 16751069.11. Nakao S, Ogtata Y, Shimizu E, Yamazaki M, Furuyama S, Sugiya H. Tumor necrosis factor alpha (TNF-alpha)-induced prostaglandin E2 release is mediated by the activation of cyclooxygenase-2 (COX-2) transcription via NFkappaB in human gingival fibroblasts. Mol Cell Biochem. 2002; 238:11–18. PMID: 12349897.12. Hallahan DE, Virudachalam S, Kuchibhotla J. Nuclearfactor kappaB dominant negative genetic constructs inhibit X-ray induction of cell adhesion molecules in the vascular endothelium. Cancer Res. 1998; 58:5484–5488. PMID: 9850083.13. Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991; 57:1–7. PMID: 2062949.14. Abe Y, Hashimoto S, Horie T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res. 1999; 39:41–47. PMID: 10051376.
Article15. Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. J Biol Chem. 1995; 270:24995–25000. PMID: 7559628.16. Camacho-Barquero L, Villegas I, Sánchez-Calvo JM, Talero E, Sánchez-Fidalgo S, Motilva V, Alarcón de la Lastra C. Curcumin, a Curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis. Int Immunopharmacol. 2007; 7:333–342. PMID: 17276891.
Article17. Shakibaei M, John T, Schulze-Tanzil G, Lehmann I, Mobasheri A. Suppression of NF-kappaB activation by curcuminleads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem Pharmacol. 2007; 73:1434–1445. PMID: 17291458.18. Wang SL, Li Y, Wen Y, Chen YF, Na LX, Li ST, Sun CH. Curcumin, a potential inhibitor of up-regulation of TNF-alpha and IL-6 induced by palmitate in 3T3-L1 adipocytes through NF-kappaB and JNK pathway. Biomed Environ Sci. 2009; 22:32–39. PMID: 19462685.
Article19. Thresiamma KC, George J, Kuttan R. Protective effect of curcumin, ellagic acid and bixin on radiation induced genotoxicity. J Exp Clin Cancer Res. 1998; 17:431–434. PMID: 10089063.20. Bhatia AL, Sharma A, Patni S, Sharma AL. Prophylactic effect of flaxseed oil against radiation-induced hepatotoxicity in mice. Phytother Res. 2007; 21:852–859. PMID: 17486687.
Article21. Lee JC, Krochak R, Blouin A, Kanterakis S, Chatterjee S, Arguiri E, Vachani A, Solomides CC, Cengel KA, Christofidou-Solomidou M. Dietary flaxseed prevents radiation-induced oxidative lung damage, inflammation and fibrosis in a mouse model of thoracic radiation injury. Cancer Biol Ther. 2009; 8:47–53. PMID: 18981722.
Article22. Lee JC, Kinniry PA, Arguiri E, Serota M, Kanterakis S, Chatterjee S, Solomides CC, Javvadi P, Koumenis C, Cengel KA, Christofidou-Solomidou M. Dietary curcumin increases antioxidant defenses in lung, ameliorates radiation-induced pulmonary fibrosis, and improves survival in mice. Radiat Res. 2010; 173:590–601. PMID: 20426658.
Article23. Venkatesan N, Punithavathi D, Babu M. Protection from acute and chronic lung diseases by curcumin. Adv Exp Med Biol. 2007; 595:379–405. PMID: 17569221.
Article24. Rezvani M, Ross GA. Modification of radiation-induced acute oral mucositis in the rat. Int J Radiat Biol. 2004; 80:177–182. PMID: 15164799.
Article25. Jagetia GC, Rajanikant GK. Effect of curcumin on radiation-impaired healing of excisional wounds in mice. J Wound Care. 2004; 13:107–109. PMID: 15045805.
Article26. Lee KH, Rhee KH. Radioprotective effect of cyclo(L-phenylalanyl-L-prolyl) on irradiated rat lung. J Microbiol Biotechnol. 2008; 18:369–376. PMID: 18309286.27. Müller JM, Ziegler-Heitbrock HW, Baeuerle PA. Nuclear factor kappa B, a mediator of lipopolysaccharide effects. Immunobiology. 1993; 187:233–256. PMID: 8330898.
Article28. Vergara JA, Raymond U, Thet LA. Changes in lung morphology and cell number in radiation pneumonitis and fibrosis: a quantitative ultrastructural study. Int J Radiat Oncol Biol Phys. 1987; 13:723–732. PMID: 3570895.29. Guerry-Force ML, Perkett EA, Brigham KL, Meyrick B. Early structural changes in sheep lung following thoracic irradiation. Radiat Res. 1988; 114:138–153. PMID: 3353501.
Article30. Nicholas D, Down JD. The assessment of early and late radiation injury to the mouse lung using X-ray computerised tomography. Radiother Oncol. 1985; 4:253–263. PMID: 4081113.
Article31. Willner J, Vordermark D, Schmidt M, Gassel A, Flentje M, Wirtz H. Secretory activity and cell cycle alteration of alveolar type II cells in the early and late phase after irradiation. Int J Radiat Oncol Biol Phys. 2003; 55:617–625. PMID: 12573748.
Article32. Coggle JE, Lambert BE, Moores SR. Radiation effects in the lung. Environ Health Perspect. 1986; 70:261–291. PMID: 3549278.
Article33. Morgan GW, Breit SN. Radiation and the lung: a reevaluation of the mechanisms mediating pulmonary injury. Int J Radiat Oncol Biol Phys. 1995; 31:361–369. PMID: 7836090.
Article34. Nishioka A, Ogawa Y, Mima T, Jin YJ, Sonobe H, Kariya S, Kubota K, Yoshida S, Ueno H. Histopathologic amelioration of fibroproliferative change in rat irradiated lung using soluble transforming growth factor-beta (TGF-beta) receptor mediated by adenoviral vector. Int J Radiat Oncol Biol Phys. 2004; 58:1235–1241. PMID: 15001268.35. Vujaskovic Z, Marks LB, Anscher MS. The physical parameters and molecular events associated with radiation-induced lung toxicity. Semin Radiat Oncol. 2000; 10:296–307. PMID: 11040330.
Article36. Kuroki M, Noguchi Y, Shimono M, Tomono K, Tashiro T, Obata Y, Nakayama E, Kohno S. Repression of bleomycin-induced pneumopathy by TNF. J Immunol. 2003; 170:567–574. PMID: 12496444.
Article37. Martinet Y, Rom WN, Grotendorst GR, Martin GR, Crystal RG. Exaggerated spontaneous release of platelet-derived growth factor by alveolar macrophages from patients with idiopathic pulmonary fibrosis. N Engl J Med. 1987; 317:202–209. PMID: 3600711.
Article38. Vignaud JM, Allam M, Martinet N, Pech M, Plenat F, Martinet Y. Presence of platelet-derived growth factor in normal and fibrotic lung is specifically associated with interstitial macrophages, while both interstitial macrophages and alveolar epithelial cells express the c-sis proto-oncogene. Am J Respir Cell Mol Biol. 1991; 5:531–538. PMID: 1958380.39. Büttner C, Skupin A, Reimann T, Rieber EP, Unteregger G, Geyer P, Frank KH. Local production of interleukin-4 during radiation-induced pneumonitis and pulmonary fibrosis in rats: macrophages as a prominent source of interleukin-4. Am J Respir Cell Mol Biol. 1997; 17:315–325. PMID: 9308918.
Article40. Yi ES, Bedoya A, Lee H, Chin E, Saunders W, Kim SJ, Danielpour D, Remick DG, Yin S, Ulich TR. Radiation-induced lung injury in vivo: expression of transforming growth factor-beta precedes fibrosis. Inflammation. 1996; 20:339–352. PMID: 8872498.
Article41. Park KJ, Oh YT, Kil WJ, Park W, Kang SH, Chun M. Bronchoalveolar lavage findings of radiation induced lung damage in rats. J Radiat Res. 2009; 50:177–182. PMID: 19377267.
Article42. Xia DH, Xi L, Xv C, Mao WD, Shen WS, Shu ZQ, Yang HZ, Dai M. The protective effects of ambroxol on radiation lung injury and influence on production of transforming growth factor beta1 and tumor necrosis factor alpha. Med Oncol. 2010; 27:697–701. PMID: 19636975.43. Sandur SK, Deorukhkar A, Pandey MK, Pabón AM, Shentu S, Guha S, Aggarwal BB, Krishnan S. Curcumin modulates the radiosensitivity of colorectal cancer cells by suppressing constitutive and inducible NF-kappaB activity. Int J Radiat Oncol Biol Phys. 2009; 75:534–542. PMID: 19735878.44. Charbeneau RP, Peters-Golden M. Eicosanoids: mediators and therapeutic targets in fibrotic lung disease. Clin Sci (Lond). 2005; 108:479–491. PMID: 15896193.
Article45. Yang HJ, Youn H, Seong KM, Yun YJ, Kim W, Kim YH, Lee JY, Kim CS, Jin YW, Youn B. Psoralidin, a dual inhibitor of COX-2 and 5-LOX, regulates ionizing radiation (IR)-induced pulmonary inflammation. Biochem Pharmacol. 2011; 82:524–534. PMID: 21669192.
Article46. Card JW, Voltz JW, Carey MA, Bradbury JA, Degraff LM, Lih FB, Bonner JC, Morgan DL, Flake GP, Zeldin DC. Cyclooxygenase-2deficiency exacerbates bleomycin-induced lung dysfunction but not fibrosis. Am J Respir Cell Mol Biol. 2007; 37:300–308. PMID: 17496151.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Histomorphologic Change of Radiation Pneumonitis in Rat Lungs : Captopril Reduces Rat Lung Injury Induced by Irradiation
- Reducing Effect of Angiotensin-1 Converting Enzyme Inhibitor (Captopril) in Fibrosis of Radiation Induced Lung Injury
- Inhibitory effects of curcumin on passive cutaneous anaphylactoid response and compound 48/80-induced mast cell activation
- Curcumin Attenuates Acrolein-induced COX-2 Expression and Prostaglandin Production in Human Umbilical Vein Endothelial Cells
- The Effect of Cis-Diammine Dichloro Platinum(II) on Radiation Injury in the Rat Bowel