J Korean Soc Endocrinol.
2006 Aug;21(4):302-310. 10.3803/jkes.2006.21.4.302.
The Effect of Epicatechin on the High Glucose-induced TSP-1 Expression and MMP-2 Activity in Rat Vascular Smooth Muscle Cells
- Affiliations
-
- 1Department of Physiology, College of Medicine, The Catholic University of Korea, Korea.
- 2Department of Biochemistry, College of Medicine, The Catholic University of Korea, Korea.
- 3Department of Internal Medicine, College of Medicine, Hallym University, Korea.
- KMID: 2200845
- DOI: http://doi.org/10.3803/jkes.2006.21.4.302
Abstract
-
BACKGROUND: The incidence of atherosclerosis is well correlated with the progression of type 2 diabetes mellitus. High plasma glucose in uncontrolled diabetic patients evokes many vascular complications such as atherosclerosis. Specifically, high glucose was reported to induce thrombospondin-1 (TSP-1), which activates matrix metalloproteinase-2 (MMP-2) and leads to the invasion of vascular smooth muscle cells (VSMCs) into the intima. Catechins with antioxidant effects are known to inhibit MMP-2 activity. Therefore, this study was aimed at revealing the effect of epicatechin, one of catechins, on high glucose-induced TSP-1 and the invasiveness of VSMCs.
METHODS
VSMCs were primarily isolated from Sprague-Dawley rat aorta. The VSMCs were incubated with different doses (30, 100 and 300 micrometer) of epicatechin under high glucose concentration (30 mM). The TSP-1 protein and mRNA expressions were analyzed by performing Western blotting and Northern blot analyses, respectively. RT-PCR was performed to observe the MMP-2 mRNA expression. Gelatin zymography was performed for the measurement of MMP-2 activity. Invasion assays were performed to evaluate the invasiveness of VSMCs.
RESULTS
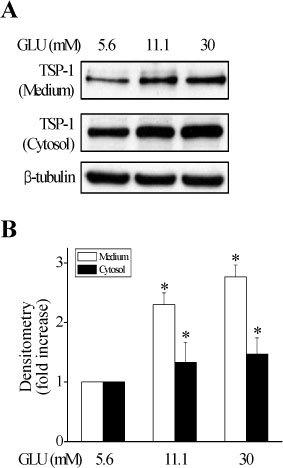
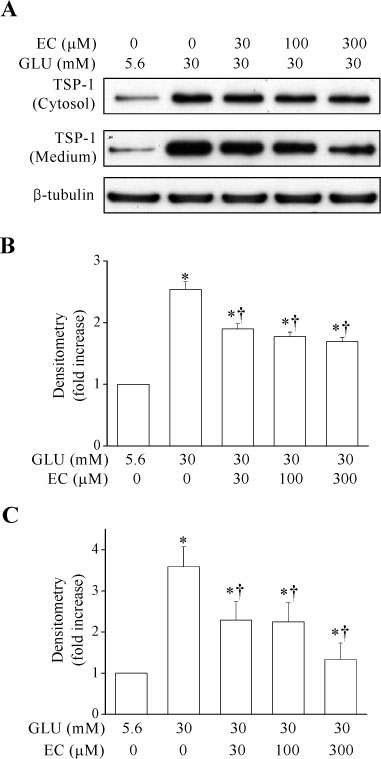
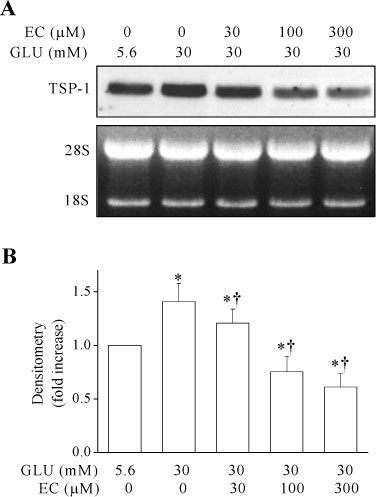
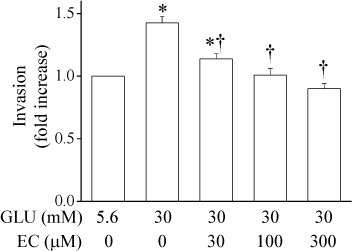
Epicatechin inhibited the high glucose-induced TSP-1 expression and the MMP-2 activity in a dose-dependent manner. Also, epicatechin inhibited the high glucose-induced invasiveness of VSMCs across the matrix barrier in a dose-dependent fashion.
CONCLUSION
Collectively, epicatechin may prevent the high glucose-induced proliferation and invasion of VSMCs by inhibiting the TSP-1 expression and the MMP-2 activity. Therefore, epicatechin appears to play a protective role in the development of atherosclerosis.
MeSH Terms
-
Animals
Antioxidants
Aorta
Atherosclerosis
Blood Glucose
Blotting, Northern
Blotting, Western
Catechin*
Diabetes Mellitus, Type 2
Gelatin
Glucose
Humans
Incidence
Matrix Metalloproteinase 2
Muscle, Smooth, Vascular*
Rats*
Rats, Sprague-Dawley
RNA, Messenger
Thrombospondin 1*
Antioxidants
Catechin
Gelatin
Glucose
Matrix Metalloproteinase 2
RNA, Messenger
Thrombospondin 1
Figure
Reference
-
1. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998. 352:837–853.2. Ruderman N, Haudenschild C. Diabetes as an atherogenic factor. Prog Cardiovasc Dis. 1984. 329:977–986.3. Graier WF, Grubenthal I, Dittrich P, Wascher TC, Kostner GM. Intracellular mechanism of high D-glucose-induced modulation of vascular cell proliferation. Eur J Pharmacol. 1995. 294:221–229.4. Pauly RR, Passaniti A, Bilato C, Monticone R, Cheng L, Papadopoulos N, Gluzband YA, Smith L, Weinstein C, Lakatta EG, Crow MT. Migration of cultured vascular smooth muscle cells through a basement membrane barrier requires type IV collagenase activity and is inhibited by cellular differentiation. Circ Res. 1994. 75:41–54.5. Mason DP, Kenagy RD, Hasenstab D, Bowen-Pope DF, Seifert RA, Coats S, Hawkins SM, Clowes AW. Matrix metalloproteinase-9 overexpression enhances vascular smooth muscle cell migration and alters remodeling in the injured rat carotid artery. Circ Res. 1999. 85:1179–1185.6. Hao F, Yu JD. High glucose enhance expression of matrix metalloproteinase-2 in smooth muscle cells. Acta Pharmacol Sin. 2003. 24:534–538.7. Baenziger NL, Brodie GN, Majerus PW. A thrombin-sensitive protein of human platelet membranes. Proc Natl Acad Sci U S A. 1971. 68:240–243.8. Lawler J. The structural and functional properties of thrombospondin. Blood. 1986. 67:1197–1209.9. Clezardin P, Lawler J, Amiral J, Quentin G, Delmas P. Identification of cell adhesive sites in the N-terminal domain of thrombospondin-1. Biochem J. 1997. 321:819–827.10. Poczatek MH, Hugo C, Darley-Usmar V, Murphy-Ullrich JE. Glucose stimulation of transforming growth factor-beta bioactivity in mesangial cells is mediated by thrombospondin-1. Am J Pathol. 2000. 157:1353–1363.11. Wang S, Skorczewski J, Feng X, Mei L, Murphy-Ullrich JE. Glucose up-regulates thrombospondin 1 gene transcription and transforming growth factor-beta activity through antagonism of cGMP-dependent protein kinase repression via upstream stimulatory factor 2. J Biol Chem. 2004. 279:34311–34322.12. Stenina OI, Krukovets I, Wang K, Zhou Z, Forudi F, Penn MS, Topol EJ, Plow EF. Increased expression of thrombospondin-1 in vessel wall of diabetic Zucker rat. Circulation. 2003. 107:3209–3215.13. Hofmann CS, Sonenshein GE. Green tea polyphenol epigallocatechiin-3 gallate induces apoptosis of proliferating vascular smooth muscle cells via activation of p53. FASEB J. 2003. 17:702–704.14. Galis ZS, Muszynski M, Sukhova GK, Simon-Morrissey E, Unemori EN, Lark MW, Amento E, Libby P. Cytokine-stimulated human vascular smooth muscle cells synthesize a complement of enzymes required for extracellular matrix digestion. Circ Res. 1994. 75:181–189.15. Esparza J, Vilardell C, Calvo J, Juan M, Vives J, Urbano-Marquez A, Yague J, Cid MC. Fibronectin upregulates gelatinase B (MMP-9) and induces coordinated expression of gelatinase A (MMP-2) and its activator MT1-MMP (MMP-14) by human T lymphocyte cell lines. A process repressed through RAS/MAP kinase signaling pathways. Blood. 1999. 94:2754–2766.16. Zempo N, Kenagy RD, Au YP, Bendeck M, Clowes MM, Reidy MA, Clowes AW. Matrix metalloproteinases of vascular wall cells are increased in balloon-injured rat carotid artery. J Vasc Surg. 1994. 20:209–217.17. Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994. 370:61–65.18. Lee T, Esemuede N, Sumpio BE, Gahtan V. Thrombospondin-1 induces matrix metalloproteinase-2 activation in vascular smooth muscle cells. J Vasc Surg. 2003. 38:147–154.19. da Silva EL, Abdalla DS, Terao J. Inhibitory effect of flavonoids on low-density lipoprotein peroxidation catalyzed by mammalian 15-lipoxygenase. IUBMB Life. 2000. 49:289–295.20. Arts IC, Hollman PC, Feskens EJ, Bueno de Mesquita HB, Kromhout D. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study. Am J Clin Nutr. 2001. 74:227–232.21. Maeda K, Kuzuya M, Cheng XW, Asai T, Kanda S, Tamaya-Mori N, Sasaki T, Shibata T, Iguchi A. Green tea catechins inhibit the cultured smooth muscle cell invasion through the basement barrier. Atherosclerosis. 2003. 166:23–30.22. Ryu GR, Kang JH, Jang HI, Ko SH, Jeong IK, Rhie DJ, Yoon SH, Hahn SJ, Jo YH, Kim MS, Kim MJ. The role of cAMP/PKA activation on exendin-4-induced cyclin D1 expression in INS-1 cell. J Kor Diabetes Assoc. 2005. 29:295–303.23. Kim MJ, Ryu GR, Kang JH, Sim SS, Min do S, Rhie DJ, Yoon SH, Hahn SJ, Jeong IK, Hong KJ, Kim MS, Jo YH. Inhibitory effects of epicatechin on interleukin-1beta-induced inducible nitric oxide synthase expression in RINm5F cells and rat pancreatic islets by down-regulation of NF-kappaB activation. Biochem Pharmacol. 2004. 68:1775–1785.24. Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, McEwan RN. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987. 47:3239–3245.25. Craig WJ. Health-promoting properties of common herbs. Am J Clin Nutr. 1999. 70:suppl 3. S491–S499.26. Guo Q, Zhao B, Shen S, Hou J, Hu J, Xin W. ESR study on the structure-antioxidant activity relationship of tea catechins and their epimers. Biochim Biophys Acta. 1999. 1427:13–23.27. Bendeck MP, Conte M, Zhang M, Nili N, Strauss BH, Farwell SM. Doxycycline modulates smooth muscle cell growth, migration, and matrix remodeling after arterial injury. Am J Pathol. 2002. 160:1089–1095.28. Taraboletti G, Morbidelli L, Donnini S, Parenti A, Granger HJ, Giavazzi R, Ziche M. The heparin binding 25 kDa fragment of thrombospondin-1 promotes angiogenesis and modulates gelatinase and TIMP-2 production in endothelial cells. FASEB J. 2000. 14:1674–1676.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Insulin Enhances Suppressive Effect of Lipopolysaccharide on Glucose-induced Proliferation of Vascular Smooth Muscle Cells
- Porphyromonas gingivalis lipopolysaccharide stimulates vascular smooth muscle cell migration through signal transducer and activator of transcription 3-mediated matrix metalloproteinase-9 expression
- alpha-Lipoic acid inhibits matrix metalloproteinase-9 expression by inhibiting NF-kappaB transcriptional activity
- Effect of Transforming Growth Factor-Induced Gene Product, beta ig-h3 on Proliferation, Migration, and Adhesion of Aortic Smooth Muscle Cells Cultured in High Glucose
- Effect of high glucose on synthesis and gene expression of collagen and fibronectin in cultured vascular smooth muscle cells