Korean J Phys Anthropol.
2016 Mar;29(1):19-26. 10.11637/kjpa.2016.29.1.19.
The Changes of Pro-inflammatory Cytokines in Serum according to the Reperfusion Time after Ischemia of Left Common Iliac Artery in Mice
- Affiliations
-
- 1Department of Anatomy and Cell Biology, College of Medicine, Hanyang University, Korea.
- 2College of Nursing, Hanyang University, Korea. seoyk75@hanyang.ac.kr
- KMID: 2161137
- DOI: http://doi.org/10.11637/kjpa.2016.29.1.19
Abstract
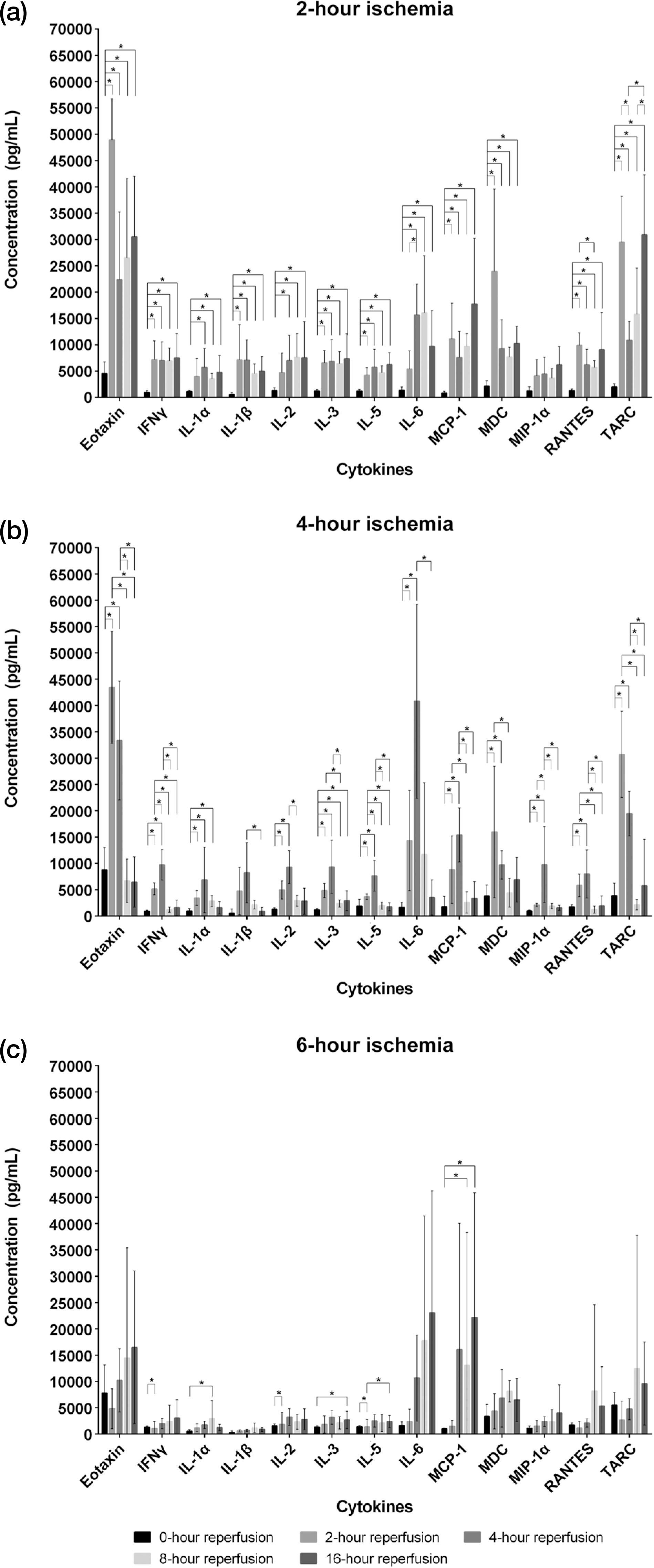
- Ischemia-reperfusion injury arises from the restoration of blood supply after ischemia. Both reactive oxygen species and various cytokines produced by activated immune cells are the primary causal risk factors for ischemic injury. Cytokines are intercellular signaling substances for regulating any infection, immune reactions and inflammation, and pro-inflammatory cytokines adversely affect any diseases through an increase in inflammatory reaction. This study was conducted to investigate whether the periods of reperfusion after ischemia result in any changes of pro-inflammatory cytokines in the serum, including IL-1α, IL-1β, IL-2, IL-3, IL-5, IL-6, Eotaxin, MCP-1, MDC, MIP-1α, RANTES, TARC, IFNδ. A total of 96 male mice aged at 12 weeks was used in this study, and the groups of ischemia were divided into the following three different groups: 2-hour, 4-hour, and 6-hour ischemia groups. For the object of ischemic injury, the left common iliac artery was clamped by vascular clamp, each ischemia group was subdivided into 5 different groups according to the periods of reperfusion: 0-, 2-, 4-, 8-, and 16-hour reperfusion time. Blood samples after general anesthesia were collected from the mice hearts, and the serum was separated from them. The concentration of pro-inflammatory cytokines (IL-1α, IL-1β, IL-2, IL-3, IL-5, IL-6, Eotaxin, MCP-1, MDC, MIP-1α, RANTES, TARC, IFNδ) in the serum was measured by ELISA, and the following results were acquired. The concentrations of the 13 pro-inflammatory cytokines were significantly different in accordance with the periods of ischemia and the reperfusion time. In 2-hour ischemia group, IL-1α and IL-3 were increaed compared to normal control group, and 12 cytokines were increased followed by reperfusion except for MIP-1α. MCP-1 and TARC were expressed as the highest concentration in the 16-hour reperfusion time. In 4-hour ischemia group, TARC was significant differences with normal control group, and the concentration of 13 cytokines were decreased after 4-hour reperfusion time. In 6-hour ischemia group, IL-2, IL-3, MCP-1 and TARC were increased, compared to normal control group, and IL-3 and MCP-1 were increased in 16-hour reperfusion time. To sum up, ischemia increased the pro-inflammatory cytokines compared to normal control group and in the 2-hour and 6-hour ischemia groups, IL-1α, IL-3, MCP-1 and TARC were increased until the late reperfusion time.
MeSH Terms
-
Anesthesia, General
Animals
Chemokine CCL5
Cytokines*
Enzyme-Linked Immunosorbent Assay
Heart
Humans
Iliac Artery*
Inflammation
Interleukin-2
Interleukin-3
Interleukin-5
Interleukin-6
Ischemia*
Male
Mice*
Reactive Oxygen Species
Reperfusion Injury
Reperfusion*
Risk Factors
Chemokine CCL5
Cytokines
Interleukin-2
Interleukin-3
Interleukin-5
Interleukin-6
Reactive Oxygen Species
Figure
Reference
-
References
1. Gillani S, Cao J, Suzuki T, Hak DJ. The effect of ischemia reperfusion injury on skeletal muscle. Injury. 2012; 43:670–5.
Article2. Kaszaki J, Wolfárd A, Szalay L, Boros M. Pathophysiology of ischemia-reperfusion Injury. Transplant Proc. 2006; 38:826–8.
Article3. Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012; 298:229–317.
Article4. Granger DN. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol. 1988; 255:H1269–75.
Article5. Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010; 10:826–37.
Article6. Zhang F, Hu EC, Gerzenshtein J, Lei MP, Lineaweaver WC. The expression of proinflammatory cytokines in the rat muscle flap with ischemia-reperfusion injury. Ann Plast Surg. 2005; 54:313–7.7. Zhang F, Hu EC, Topp S, Lei M, Chen W, Lineaweaver WC. Proinflammatory cytokines gene expression in skin flaps with arterial and venous ischemia in rats. J Reconstr Microsurg. 2006; 22:641–7.
Article8. Dinarello CA. Proinflammatory cytokines. Chest. 2000; 118:503–8.
Article9. Koh H-J. Joo J, Cho M-L, Her Y-M, Hwang J-E, Lee J. Proinflammatory and antiinflammatory cytokine balance in patients with cirrhotic hepatitis during live-donor liver transplant. Exp Clin Transplant. 2013; 11:39–43.10. Lange M, Nakano Y, Traber DL, Hamahata A, Traber LD, Enkhbaatar P. Time course of the inflammatory and oxidative stress response to pulmonary infection in mice. Exp Lung Res. 2012; 38:157–63.
Article11. Ohtsuka M, Takano H, Zou Y, Toko H, Akazawa H, Qin Y, et al. Cytokine therapy prevents left ventricular remodeling and dysfunction after myocardial infarction through neovascularization. FASEB J. 2004; 18:851–3.
Article12. Gravante G, Ong S, Metcalfe M, Sorge R, Sconocchia G, Orlando G, et al. Cytokine response to ischemia/reperfusion injury in an ex vivo perfused porcine liver model. Transplant Proc. 2009; 41:1107–12.
Article13. Yassin MM, Harkin DW, Barros D'Sa AA, Halliday MI, Rowlands BJ. Lower limb ischemia-reperfusion injury triggers a systemic inflammatory response and multiple organ dysfunction. World J Surg. 2002; 26:115–21.14. Krishnadasan B, Naidu BV, Byrne K, Fraga C, Verrier ED, Mulligan MS. The role of proinflammatory cytokines in lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2003; 125:261–72.
Article15. Zhang Z, Pan C, Wang HZ, Li YX. Protective effects of osthole on intestinal ischemia-reperfusion injury in mice. Exp Clin Transplant. 2014; 12:246–52.16. Serrick C, La Franchesca S, Giaid A, Shennib H. Cytokine interleukin-2, tumor necrosis factor-alpha, and interferon-gamma release after ischemia/reperfusion injury in a novel lung autograft animal model. Am J Respir Crit Care Med. 1995; 152:277–82.
Article17. Auclair S, Roth K, Saunders B, Ogborn K, Sheikh A, Naples J, et al. Interleukin-3-deficient mice have increased resistance to blood-stage malaria. Infect Immun. 2014; 192:1308–14.
Article18. Kamenetz Y, Beloosesky Y, Zeltzer C, Gotlieb D, Magaza-nik A, Fishman P, et al. Relationship between routine hematological parameters, serum IL-3, IL-6 and erythropoietin and mild anemia and degree of function in the elderly. Aging (Milano). 1998; 10:32–8.
Article19. Meldrum DR, Ayala A, Perrin MM, Ertel W, Chaudry IH. Diltiazem restores IL-2, IL-3, IL-6, and IFN-γ synthesis and decreases host susceptibility to sepsis following hemorrhage. J Surg Res. 1991; 51:158–64.
Article20. Xu YX, Wichmann MW, Ayala A, Cioffi WG, Chaudry IH. Trauma-hemorrhage induces increased thymic apoptosis while decreasing IL-3 release and increasing GM-CSF. J Surg Res. 1997; 68:24–30.
Article21. Sanderson CJ. Interleukin-5, eosinophils, and disease. Blood. 1992; 79:3101–9.
Article22. Li S, Zhu FX, Zhang HB, Li H, An YZ. Pretreatment with interleukin-33 reduces warm hepatic ischemia/reperfusion injury in mice. Chin Med J (Engl). 2013; 126:1855–9.23. Clementsen T, Reikeras O. Cytokine patterns after tourniquet-induced skeletal muscle ischaemia reperfusion in total knee replacement. Scand J Clin Lab Invest. 2008; 68:154–9.
Article24. Seekamp A, Warren JS, Remick DG, Till GO, Ward PA. Requirements for tumor necrosis factor-alpha and interleukin-1 in limb ischemia/reperfusion injury and associated lung injury. Am J Pathol. 1993; 143:453–63.25. Roy S, Das S, Munshi A, Kaul S, Jyothy A. Association of −1382A>G CCL11 gene variant with ischemic stroke, its subtypes and hemorrhagic stroke in a South Indian population. Neurol India. 2014; 62:387–92.26. Nishi T, Maier CM, Hayashi T, Saito A, Chan PH. Superoxide dismutase 1 overexpression reduces MCP-1 and MIP-1 alpha expression after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2005; 25:1312–24.27. Ramos CD, Canetti C, Souto JT, Silva JS, Hogaboam CM, Ferreira SH, et al. MIP-1alpha [CCL3] acting on the CCR1 receptor mediates neutrophil migration in immune inflammation via sequential release of TNF-alpha and LTB4. J Leukoc Biol. 2005; 78:167–77.28. Naidu BV, Farivar AS, Woolley SM, Grainger D, Verrier ED, Mulligan MS. Novel broad-spectrum chemokine inhibitor protects against lung ischemia-reperfusion injury. J Heart Lung Transplant. 2004; 23:128–34.
Article29. Richter JR, Sutton JM, Belizaire RM, Friend LA, Schuster RM, Johannigman TA, et al. Macrophage-derived chemokine (CCL22) is a novel mediator of lung inflammation following hemorrhage and resuscitation. Shock. 2014; 42:525–31.
Article30. Marques RE, Guabiraba R, Russo RC, Teixeira MM. Targeting CCL5 in inflammation. Expert Opin Ther Targets. 2013; 17:1439–60.
Article31. Levy JA. The unexpected pleiotropic activities of RANTES. J Immunol. 2009; 182:3945–6.
Article32. Ritter M, Goggel R, Chaudhary N, Wiedenmann A, Jung B, Weith A, et al. Elevated expression of TARC (CCL17) and MDC (CCL22) in models of cigarette smoke-induced pulmonary inflammation. Biochem Biophys Res Commun. 2005; 334:254–62.
Article33. Greaves DR, Häkkinen T, Lucas AD, Liddiard K, Jones E, Quinn CM, et al. Linked chromosome 16q13 chemokines, macrophage-derived chemokine, fractalkine, and thymus and activation-regulated chemokine are expressed in human atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2001; 21:923–9.
Article34. Burne MJ, Daniels F, EI Ghandour A, Mauiyyedi S, Colvin RB, O'Donnell MP, et al. Identification of the CD4+ T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest. 2001; 108:1283–90.35. Daemen MA, van't Veer C, Wolfs TG, Buurman WA. Ischemia/reperfusion-induced IFN-gamma upregulation: involvement of IL-12 and IL-18. J Immunol. 1999; 162:5506–10.36. Sukhotnik I, Miselevich I, Lurie M, Nativ O, Coran AG, Mogilner JG. The time relationship between ipsilateral testicular ischemia and germ cell apoptosis in the contralateral testis in rat. Pediatr Surg Int. 2005; 21:512–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Changes of Pro- and Anti-inflammatory Cytokines in Both Serum and Gastrocnemius Muscle of Mice after 2-hour Postischemic Reperfusion Injury
- The Effect of Ischemic Preconditioning on the Expression of Serum Cytokines after 2 Hours Ischemia and Timely Reperfusion in the Hindlimb of Mice
- Expressions of the Proinflammatory Cytokines in Rat Kidney with Cyclic Episodes of Short Ischemia-reperfusion of Left Common Iliac Artery
- An Experimental Study in Mice for Inducing the Optimal Hepatic Ischemia Followed by Reperfusion
- The Role of Adiponectin in Secondary Inflammatory Reaction in Cerebral Ischemia