J Cerebrovasc Endovasc Neurosurg.
2013 Sep;15(3):171-176. 10.7461/jcen.2013.15.3.171.
The Role of Adiponectin in Secondary Inflammatory Reaction in Cerebral Ischemia
- Affiliations
-
- 1Department of Neurosurgery, Korea University Ansan Hospital, School of Medicine, Korea University, Ansan, Korea. sungkha@yahoo.com
- KMID: 1491435
- DOI: http://doi.org/10.7461/jcen.2013.15.3.171
Abstract
OBJECTIVE
In this study, we investigate the role of adiponectin in the interaction between leukocytes and endothelium in the secondary inflammatory reaction of cerebral ischemia.
METHODS
Adiponectin knock-out mice group (APN-KO) (n = 8) and wild-type mice group (WT) (n = 8) were prepared. Each group was sub-divided into 2 groups by reperfusion time. One-hour middle cerebral artery occlusion and reperfusion were induced using the intraluminal filament technique. At 6 and 12 hours after the occlusion, the mice were placed on a stereotactic frame to perform craniotomy in the left parietal area. After craniotomy, a straight pial venule was selected as a target vessel. With the fluorescence intravital microscope, the number of rolling leukocytes and leukocytes that adhered to endothelium were counted and documented at 6 and 12 hours after the reperfusion.
RESULTS
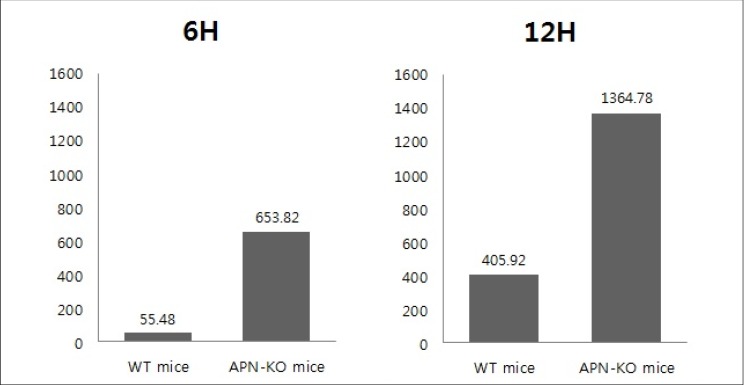
At 6 and 12 hours after the reperfusion, more rolling leukocyte and leukocyte adhesion were observed in the APN-KO mice than in the WT mice. The difference in leukocyte numbers between the APN-KO and WT mice was found to be statistically significant (p = 0.029) by Mann-Whitney U-test.
CONCLUSION
We found that adiponectin inhibits the interaction between the endothelium and leukocytes in cerebral ischemia-reperfusion. Therefore adiponectin might prevent the secondary insult caused by the inflammation reaction.
MeSH Terms
Figure
Reference
-
1. Bang OY, Saver JL, Ovbiagele B, Choi YJ, Yoon SR, Lee KH. Adiponectin levels in patients with intracranial atherosclerosis. Neurology. 2007; 5. 68(22):1931–1937. PMID: 17536050.
Article2. Barone FC, Hillegass LM, Price WJ, White RF, Lee EV, Feuerstein GZ, et al. Polymorphonuclear leukocyte infiltration into cerebral focal ischemic tissue: Myeloperoxidase activity assay and histologic verification. J Neurosci Res. 1991; 7. 29(3):336–345. PMID: 1656059.
Article3. Chen H, Chopp M, Zhang RL, Bodzin G, Chen Q, Rusche JR, et al. Anti-CD11b monoclonal antibody reduces ischemic cell damage after transient focal cerebral ischemia in rat. Ann Neurol. 1994; 4. 35(4):458–463. PMID: 8154873.
Article4. Chen MP, Tsai JC, Chung FM, Yang SS, Hsing LL, Shin SJ, et al. Hypoadiponectinemia is associated with ischemic cerebrovascular disease. Arterioscler Thromb Vasc Biol. 2005; 4. 25(4):821–826. PMID: 15692106.
Article5. Chopp M, Zhang RL, Chen H, Li Y, Jiang N, Rusche JR. Postischemic administration of an anti-Mac-1 antibody reduces ischemic cell damage after transient middle cerebral artery occlusion in rats. Stroke. 1994; 4. 25(4):869–875. discussion 875-6. PMID: 8160235.
Article6. Efstathiou SP, Tsioulos DI, Tsiakou AG, Gratsias YE, Pefanis AV, Mountokalakis TD. Plasma adiponectin levels and five-year survival after first-ever ischemic stroke. Stroke. 2005; 9. 36(9):1915–1919. PMID: 16109902.
Article7. Grau AJ, Graf T, Hacke W. Altered influence of polymorphonuclear leukocytes on coagulation in acute ischemic stroke. Thromb Res. 1994; 12. 76(6):541–549. PMID: 7900101.
Article8. Grøgaard B, Schurer L, Gerdin B, Arfors KE. Delayed hypoperfusion after incomplete forebrain ischemia in the rat. The role of polymorphonuclear leukocytes. J Cereb Blood Flow Metab. 1989; 8. 9(4):500–505. PMID: 2738115.
Article9. Hallenbeck JM. Significance of the inflammatory response in brain ischemia. Acta Neurochir Suppl. 1996; 66:27–31. PMID: 8780793.
Article10. Hallenbeck JM, Dutka AJ, Tanishima T, Kochanek PM, Kumaroo KK, Thompson CB, et al. Polymorphonuclear leukocyte accumulation in brain regions with low blood flow during the early postischemic period. Stroke. 1986; Mar-Apr. 17(2):246–253. PMID: 3961835.
Article11. Hara H, Friedlander RM, Gagliardini V, Ayata C, Fink K, Huang Z, et al. Inhibition of interleukin 1beta converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc Natl Acad Sci U S A. 1997; 3. 94(5):2007–2012. PMID: 9050895.12. Matsuo Y, Kihara T, Ikeda M, Ninomiya M, Onodera H, Kogure K. Role of neutrophils in radical production during ischemia and reperfusion of the rat brain: Effect of neutrophil depletion on extracellular ascorbyl radical formation. J Cereb Blood Flow Metab. 1995; 11. 15(6):941–947. PMID: 7593354.
Article13. Matsuo Y, Onodera H, Shiga Y, Shozuhara H, Ninomiya M, Kihara T, et al. Role of cell adhesion molecules in brain injury after transient middle cerebral artery occlusion in the rat. Brain Res. 1994; 9. 656(2):344–352. PMID: 7820595.
Article14. Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004; 1. 24(1):29–33. PMID: 14551151.
Article15. Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002; 11. 106(22):2767–2770. PMID: 12451000.
Article16. Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, et al. Novel modulator for endothelial adhesion molecules: Adipocyte-derived plasma protein adiponectin. Circulation. 1999; 12. 100(25):2473–2476. PMID: 10604883.17. Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003; 12. 14(6):561–566. PMID: 14624132.
Article18. Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004; 12. 10(12):1384–1389. PMID: 15558058.
Article19. Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem. 2004; 7. 279(27):28670–28674. PMID: 15123726.
Article20. Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005; 10. 11(10):1096–1103. PMID: 16155579.
Article21. Suzuki H, Abe K, Tojo SJ, Kitagawa H, Kimura K, Mizugaki M, et al. Reduction of ischemic brain injury by anti-P-selectin monoclonal antibody after permanent middle cerebral artery occlusion in rat. Neurol Res. 1999; 4. 21(3):269–276. PMID: 10319335.
Article22. Tao L, Jiao X, Gao E, Lau WB, Yuan Y, Lopez B, et al. Nitrative inactivation of thioredoxin-1 and its role in postischemic myocardial apoptosis. Circulation. 2006; 9. 114(13):1395–1402. PMID: 16966583.
Article23. Vasthare US, Heinel LA, Rosenwasser RH, Tuma RF. Leukocyte involvement in cerebral ischemia and reperfusion injury. Surg Neurol. 1990; 4. 33(4):261–265. PMID: 2326731.
Article24. Yatomi K, Miyamoto N, Komine-Kobayashi M, Liu M, Oishi H, Arai H, et al. Pathophysiological dual action of adiponectin after transient focal ischemia in mouse brain. Brain Res. 2009; 11. 1297:169–176. PMID: 19699724.
Article25. Zhang RL, Chopp M, Jiang N, Tang WX, Prostak J, Manning AM, et al. Anti-intercellular adhesion molecule-1 antibody reduces ischemic cell damage after transient but not permanent middle cerebral artery occlusion in the Wistar rat. Stroke. 1995; 8. 26(8):1438–1442. discussion 1443. PMID: 7631350.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Indomethacin on Ischemia-induced Brain Injury
- The Role of Plasma Adiponectin and Polymorphism of Adiponectin Gene in the Development of Type 2 Diabetes Mellitus
- The Role of Polymorphism of Adiponectin Gene in the Atherosclerosis
- Improved ovarian adiponectin system expression in polycystic ovary syndrome treated with exenatide
- Plasma Levels of Soluble Adhesion Molecules in Patients with Acute Cerebral Ischemic Stroke