Korean J Phys Anthropol.
2016 Jun;29(2):53-60. 10.11637/kjpa.2016.29.2.53.
The Effect of Ischemic Preconditioning on the Expression of Serum Cytokines after 2 Hours Ischemia and Timely Reperfusion in the Hindlimb of Mice
- Affiliations
-
- 1Department of Anatomy and Cell Biology, College of Medicine, Hanyang University, Korea.
- 2College of Nursing, Hanyang University, Korea.
- 3Department of Physical Therapy, Ansan University, Korea. sykim@ansan.ac.kr
- KMID: 2328101
- DOI: http://doi.org/10.11637/kjpa.2016.29.2.53
Abstract
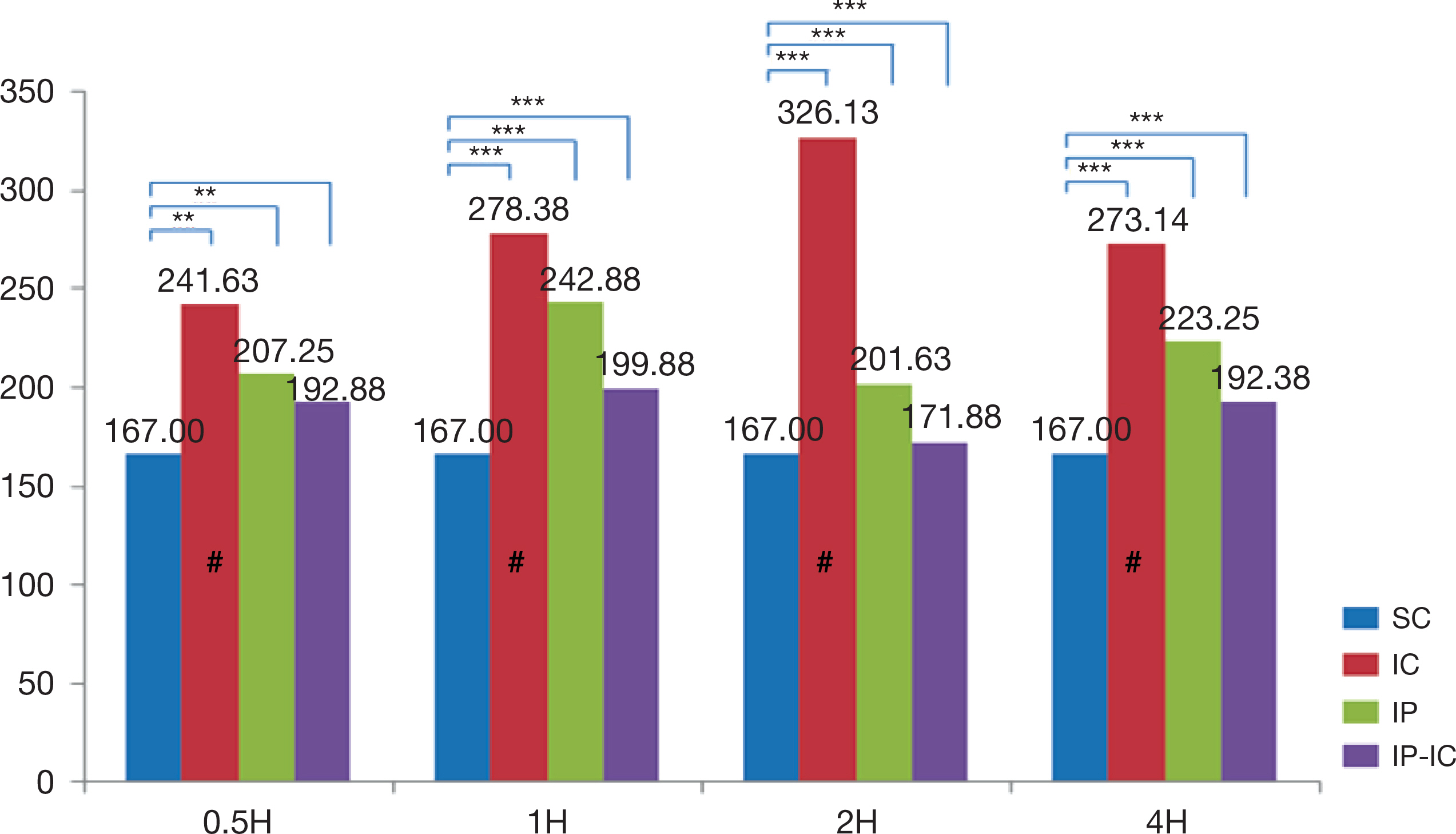
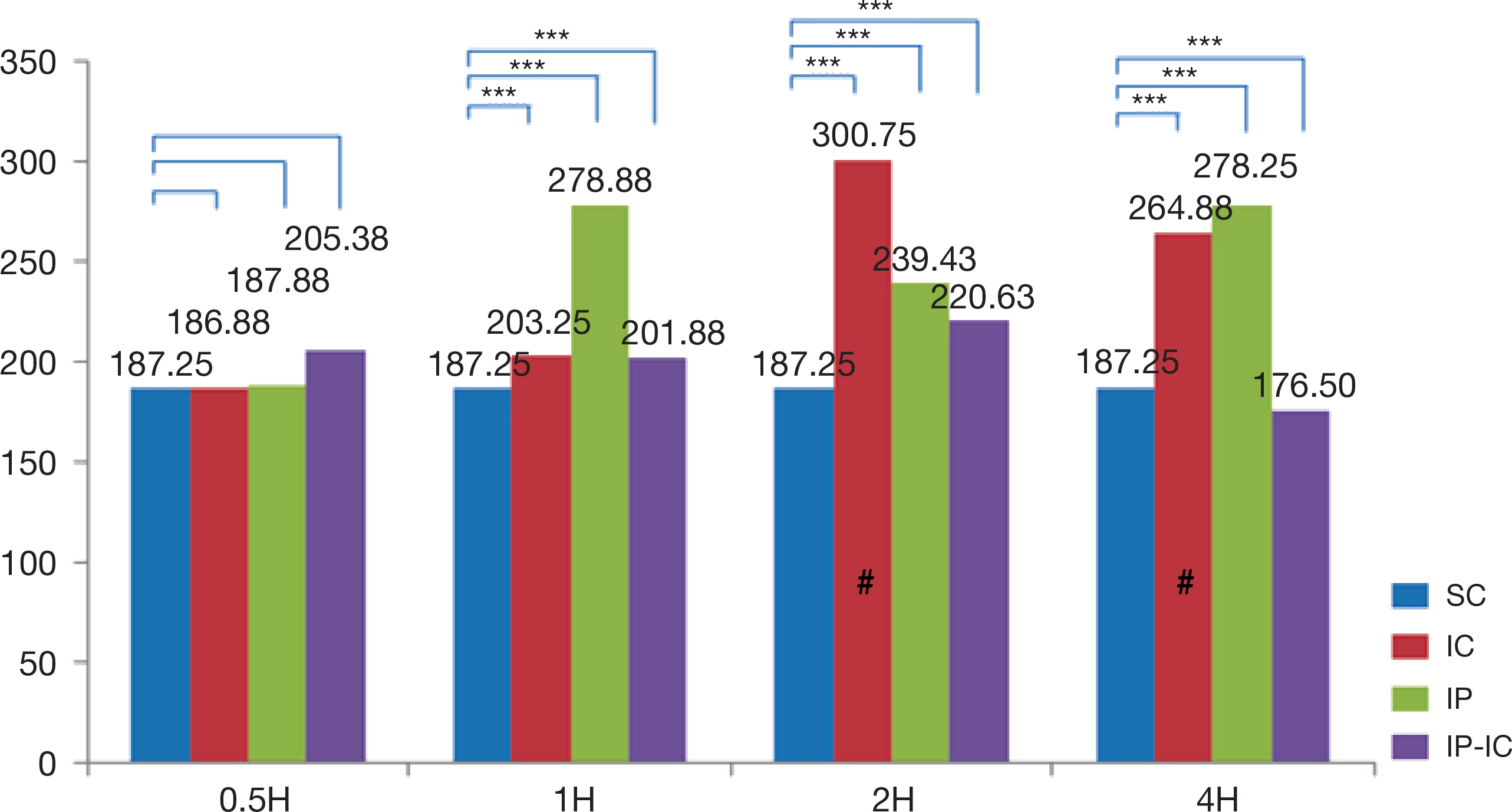
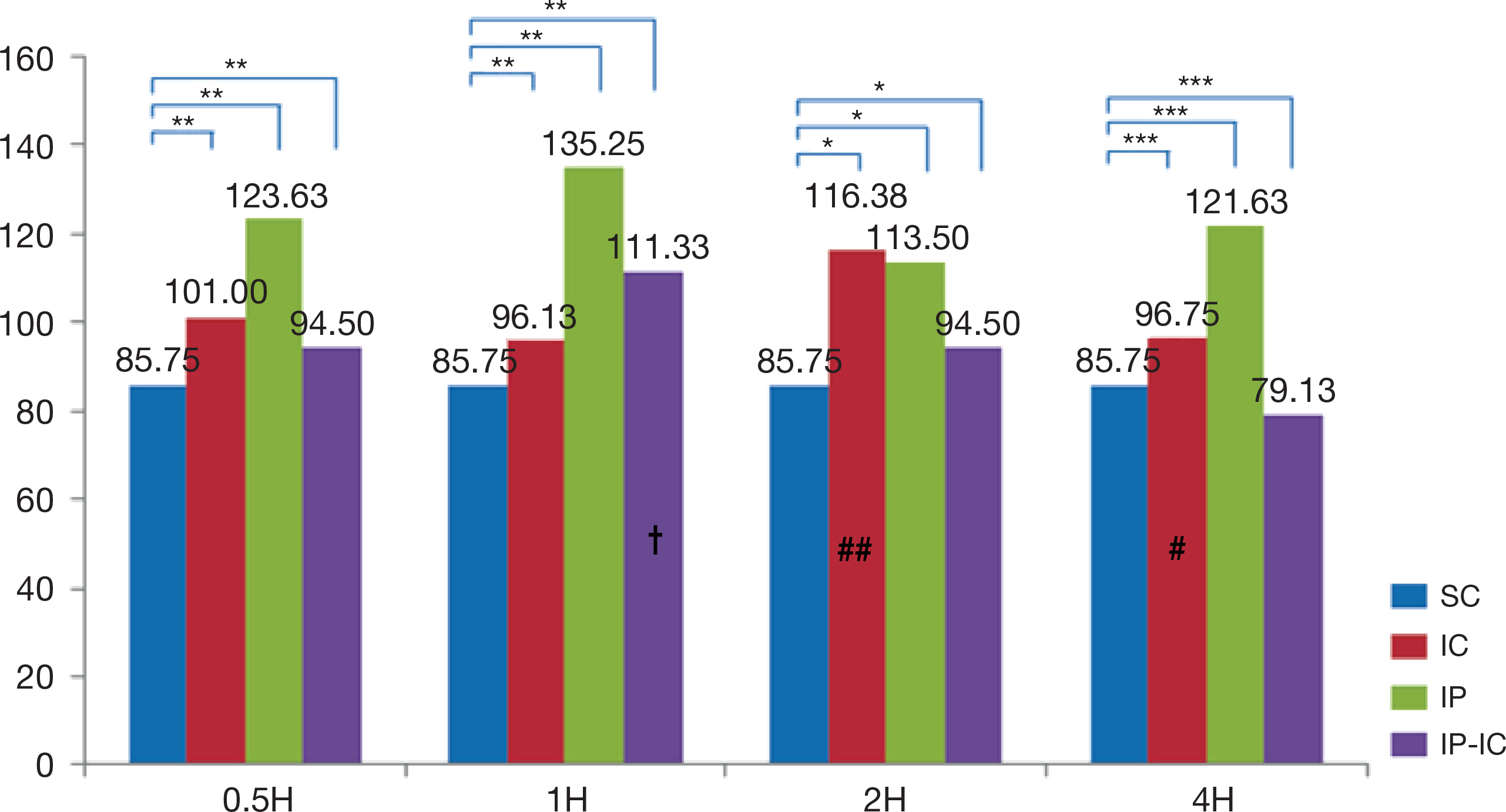
- The large volume of reactive oxygen species are generated during reperfusion after transient or post-procedural ischemia, which leads to cell injury. This ischemia-reperfusion (IR) injury may cause local and even systemic injuries. Thus, the need to reduce the IR injury has been highlighted and in this regard studies have demonstrated the ischemic preconditioning (IP) in which short ischemia and reperfusion are repeated before ischemia. Such IP is known to protect the tissues from IR injury by reducing inflammation response during ischemia. Thus, this study was based on IP known to protect the tissue with developing the mechanism of resistance to ischemia and reperfusion injury in cellular tissue. As the substance that plays an important role in the inflammatory response during IR injury is cytokines, this study was intended to review and discuss the methodologies of IP as well as to analyze the correlation of its effects on the expression of cytokines. Left common iliac artery in male mice of which weight was from 40 g to 45 g, was treated for ischemia. The animal groups consisted of ischemia (IC) group receiving 2-hour ischemic treatment alone; IP group receiving short 5-minute ischemia and reperfusion treatments repeated three times; and, ischemic preconditioning-ischemia (IP-IC) group receiving IP treatment followed by 2-hour ischemic treatment. Following these treatments in each group, reperfusion for intergroup comparisons was carried out at 30 minutes, and 1, 2 and 4 hours. The results of this study were as follows: First, the expression level of pro-inflammatory cytokines, IL-1β was the highest in IC group receiving 2-hour ischemic treatment alone (p<.001). Second, the expression level of anti-inflammatory cytokine, IL-4 was the highest in the IP group (p<.001). Third, the expression level of anti-inflammatory cytokine, IL-10 was the highest in the IP group (p<.001). In conclusion, even though the results had the degree of difference, the expression level of pro-inflammation cytokine, IL-1β in IC group was significantly lower than that in IP group, and the expression levels of anti-inflammatory cytokines, IL-4 and IL-10 in IP were significantly higher than those in IC group and IP-IC group.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Peralta C, Prats N, Xaus C, Gelpí E, Roselló-Catafau J. Protective effect of liver ischemic preconditioning on liver and lung injury induced by hepatic ischemia-reperfusion in the rat. Hepatology. 1999; 30:1481–9.
Article2. Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004; 70:71–86.
Article3. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986; 74:1124–36.
Article4. Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012; 298:229–317.
Article5. Dinarello CA. Proinflammatory cytokines. Chest Journal. 2000; 118:503–8.
Article6. Epstein FH, Luster AD. Chemokines-chemotactic cytokines that mediate inflammation. N Engl J Med. 1998; 338:436–45.7. Zhang F, Hu EC, Topp S, Lei M, Chen W, Lineaweaver WC. Proinflammatory cytokines gene expression in skin flaps with arterial and venous ischemia in rats. J Reconstr Microsurg. 2006; 22:641–7.
Article8. Zhang Z, Pan C, Wang Hz, Li YX. Protective effects of osthole on intestinal ischemia-reperfusion injury in mice. Exp Clin Transplant. 2014; 12:246–52.9. Paul We. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991; 77:1859–70.10. Engles RE, Huber TS, Zander DS, Hess PJ, Welborn MB, Moldawer LL, et al. Exogenous human recombinant interleukin-10 attenuates hindlimb ischemia-reperfusion injury. J Surg Res. 1997; 69:425–8.
Article11. Romagnani S. Biology of human TH1 and TH2 cells. J Clin Immunol. 1995; 15:121–9.
Article12. Camargo JF, Correa PA, Castiblanco J, Anaya JM. Interleukin-1beta polymorphisms in Colombian patients with autoimmune rheumatic diseases. Genes Immun. 2004; 5:609–14.13. Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: Signaling mechanisms and biologic functions. Annu Rev Immunol. 1999; 17:701–38.
Article14. Moser R, Fehr J, Bruijnzeel PL. IL-4 controls the selective endothelium-driven transmigration of eosinophils from allergic individuals. J Immunol. 1992; 149:1432–8.15. Wei M, Kuukasj Rvi P, Laurikka J, Pehkonen E, Kaukinen S, Laine S, et al. Cytokine responses in patients undergoing coronary artery bypass surgery after ischemic preconditioning. Scand Cardiovasc J. 2001; 35:142–6.16. Zhai QH, Futrell N, Chen FJ. Gene expression of IL-10 in relationship to TNF-alpha, IL-1beta and IL-2 in the rat brain following middle cerebral artery occlusion. J Neurol Sci. 1997; 152:119–24.17. Abu Amara M, Yang SY, Quaglia A, Rowley P, Tapuria N, Seifalian M, et al. Effect of remote ischemic preconditioning on liver ischemia/reperfusion injury using a new mouse model. Liver Transpl. 2011; 17:70–82.18. Takhtfooladi MA, Jahanshahi A, Sotoudeh A, Jahanshahi G, Takhtfooladi HA, Aslani K. Effect of tramadol on lung injury induced by skeletal muscle ischemia-reperfusion: an experimental study. J Bras Pneumol. 2013; 39:434–9.
Article19. Serafín A, Roselló-Catafau J, Prats N, Gelpí E, Rodés J, Peralta C. Ischemic preconditioning affects interleukin release in fatty livers of rats undergoing ischemia/reperfusion. Hepatology. 2004; 39:688–98.
Article20. Pera J, Zawadzka M, Kaminska B, Szczudlik A. Influence of chemical and ischemic preconditioning on cytokine expression after focal brain ischemia. J Neurosci Res. 2004; 78:132–140.
Article21. Kambayashi T, Jacob CO, Strassmann G. IL-4 and IL-13 modulate IL-10 release in endotoxin-stimulated murine peritoneal mononuclear phagocytes. Cell Immunol. 1996; 171:153–8.
Article22. Hart PH, Vitti GF, Burgess DR, Whitty GA, Piccoli DS, Hamilton JA. Potential antiinflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc Natl Acad Sci USA. 1989; 86:3803–7.
Article23. Kim DW, Lee JC, Cho JH, Park JH, Ahn JH, Chen BH, et al. Neuroprotection of ischemic preconditioning is mediated by antiinflammatory, not proinflammatory, cytokines in the Gerbil hippocampus induced by a subsequent lethal transient cerebral ischemia. Neurochem Res. 2015; 40:1984–95.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Ischemic Preconditioning, Adenosine and Pinacidil on Expression of Apoptosis in the Rectus Femoris Muscles of the Rats after Ischemia and Timely Reperfusion
- Changes in HO-1, HSP70 and iNOS Expressions in the Rat Liver after Remote Ischemic Preconditioning
- Effects of Ischemic Preconditioning,Adenosine and Pinacidil on the Changes in Immunoreactivities of Cu,Zn - and Mn - SOD in the Rectus Femoris Muscles of the Rats after Ischemia and Timely Reperfusion
- Expressions of the Proinflammatory Cytokines in Rat Kidney with Cyclic Episodes of Short Ischemia-reperfusion of Left Common Iliac Artery
- Effects of Ischemic Preconditioning, Adenosine and Pinacidil on the Expression of Cu,Zn- and Mn-SOD mRNA in the Rectus Femoris Muscle of the Rat after Ischemia and Timely Reperfusion