Chonnam Med J.
2016 Jan;52(1):45-52. 10.4068/cmj.2016.52.1.45.
Modulation of Melanogenesis by Heme Oxygenase-1 via p53 in Normal Human Melanocytes
- Affiliations
-
- 1Department of Dermatology, Chonnam National University Medical School, Gwangju, Korea. sjyun@chonnam.ac.kr
- KMID: 2152655
- DOI: http://doi.org/10.4068/cmj.2016.52.1.45
Abstract
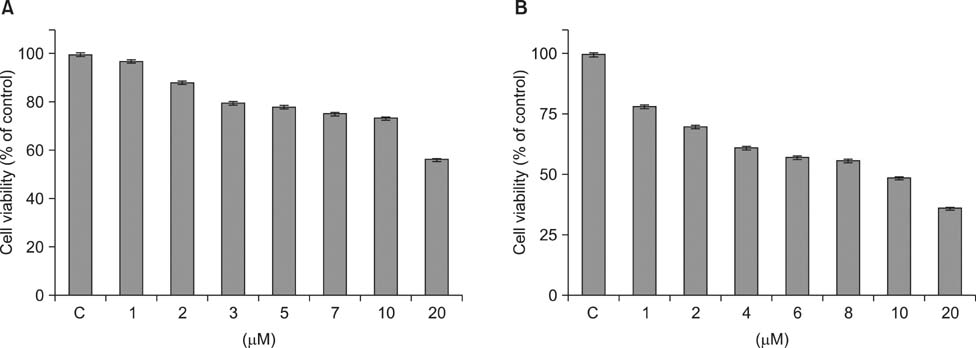
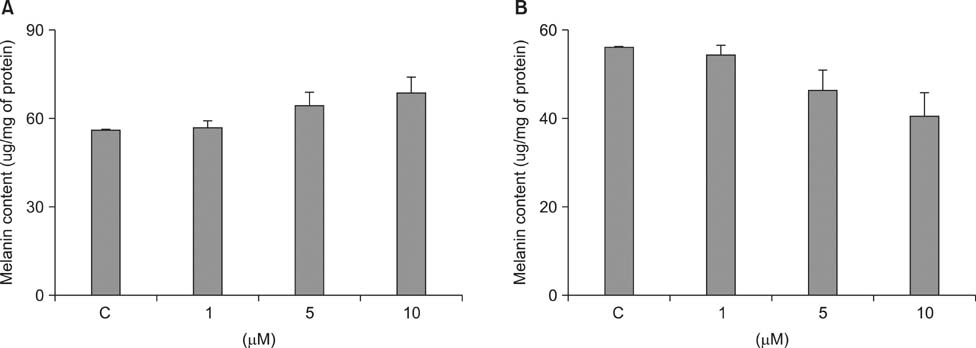
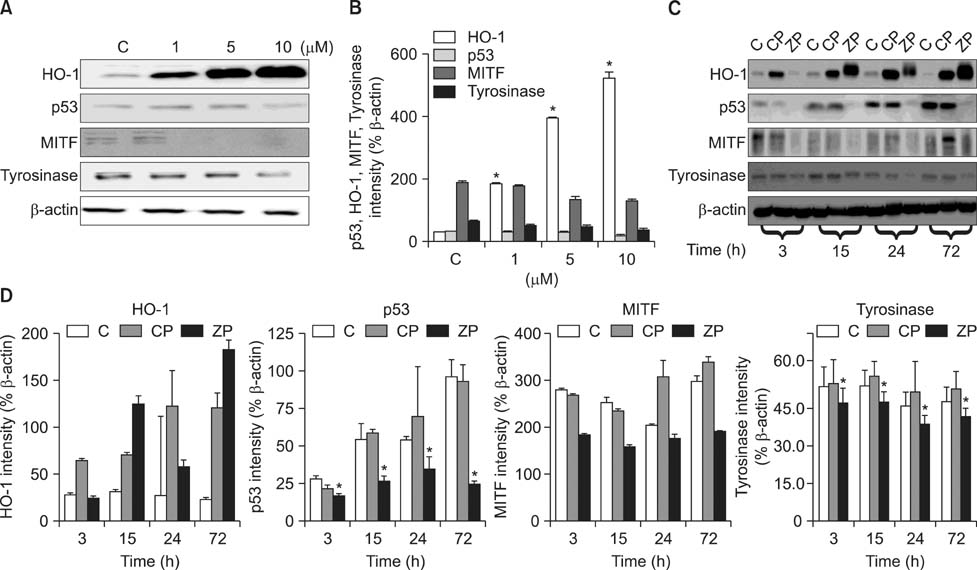
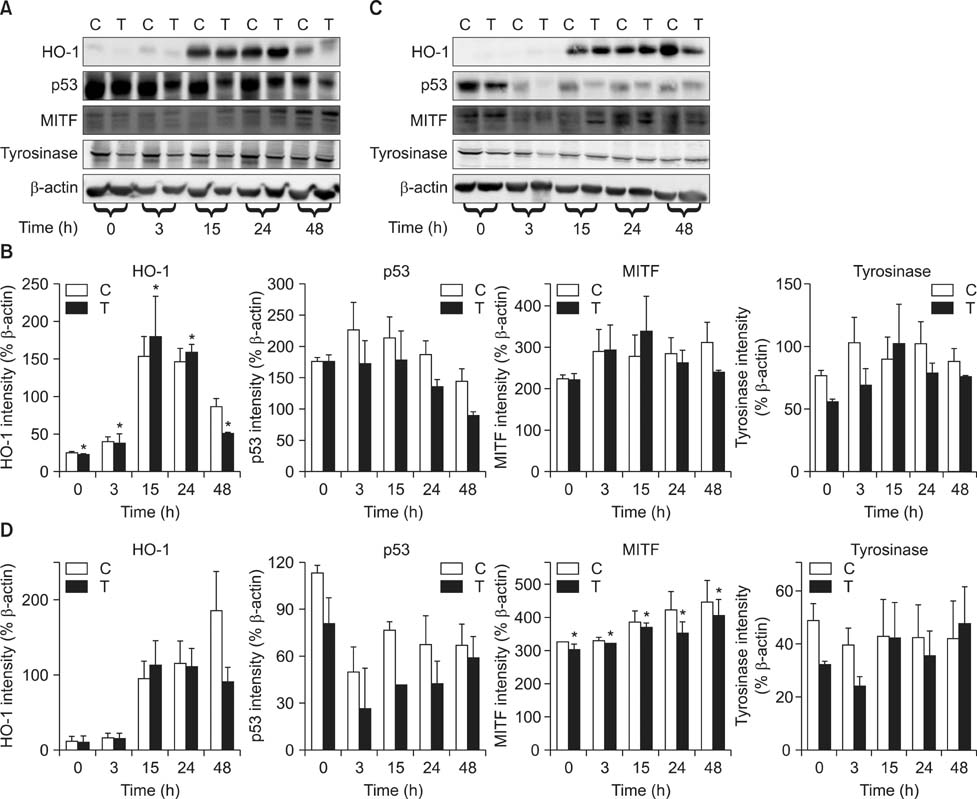
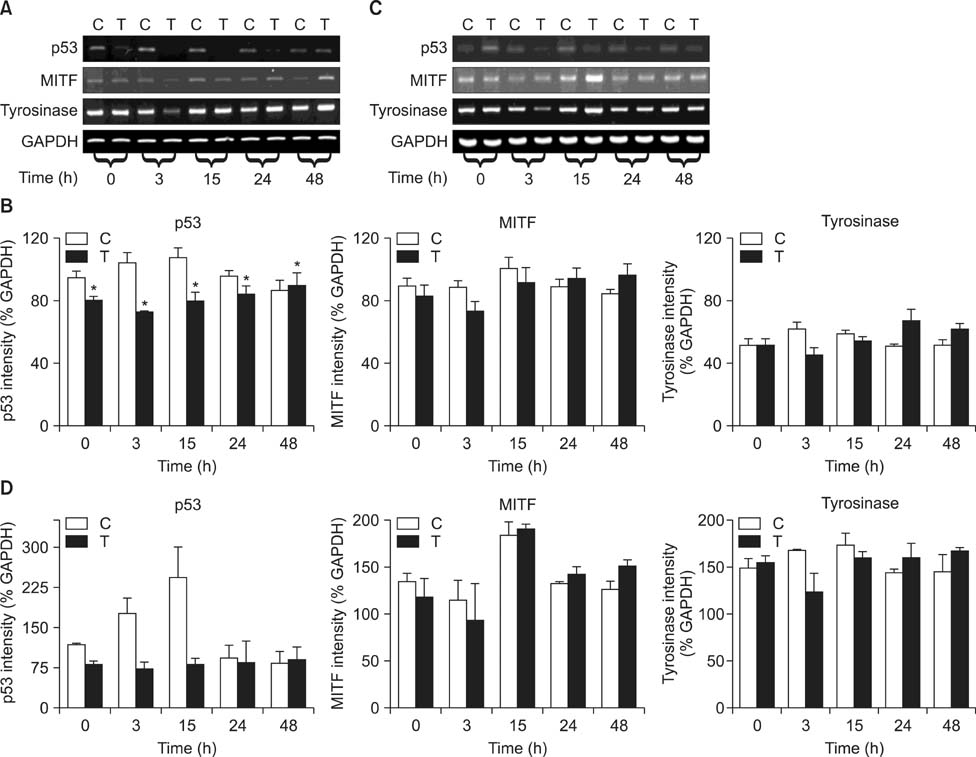
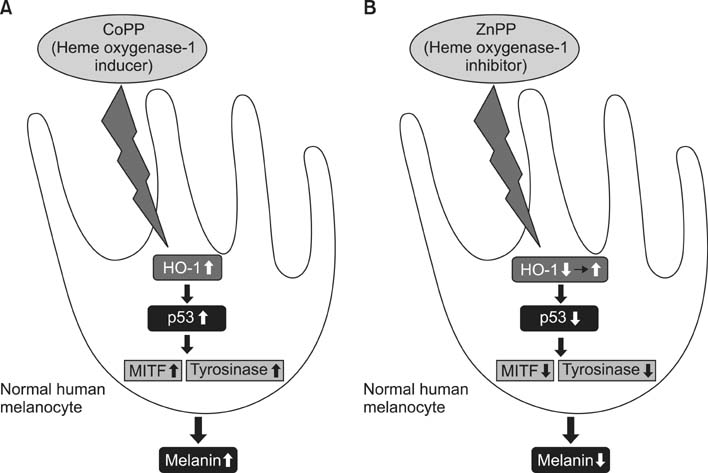
- As a key regulator of melanogenesis, p53 controls microphthalmia-associated transcription factor (MITF) and tyrosinase expression. The anti-oxidant enzyme heme oxygenase-1 (HO-1) is induced by various forms of cellular stress and diverse oxidative stimuli. However, few studies have examined the role of HO-1 in melanogenesis. Therefore, the aim of this study was to determine the role of HO-1 in melanogenesis and the mechanism underlying this relationship. Cultures of normal human melanocytes were treated with the HO-1 inducer cobalt protoporphyrin (CoPP) or the HO-1 inhibitor zinc protoporphyrin (ZnPP). We then measured the melanin content of the cells. Additional analyses consisted of Western blotting and RT-PCR. The results showed that the cellular melanin content was increased by CoPP and decreased by ZnPP. The Western blot and RT-PCR analyses showed that CoPP increased p53, MITF and tyrosinase levels, and ZnPP reduced all of them. The knockdown of p53 by siRNA transfection was followed by large decreases in the expression levels of p53, MITF and tyrosinase at 3 h of transfection. The presence of CoPP or ZnPP had no significant increased or decreased effects on MITF and tyrosinase levels from 15 h in the siRNA transfectants. Our results suggest that HO-1 modulates melanogenesis in human melanocytes via a p53-dependent pathway.
MeSH Terms
-
Blotting, Western
Cobalt
Heme Oxygenase-1*
Heme*
Humans*
Melanins
Melanocytes*
Microphthalmia-Associated Transcription Factor
Monophenol Monooxygenase
RNA, Small Interfering
Transfection
Zinc
Cobalt
Heme
Heme Oxygenase-1
Melanins
Microphthalmia-Associated Transcription Factor
Monophenol Monooxygenase
RNA, Small Interfering
Zinc
Figure
Reference
-
1. Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, et al. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest. 1999; 104:1631–1639.
Article2. Schallreuter KU, Kothari S, Chavan B, Spencer JD. Regulation of melanogenesis--controversies and new concepts. Exp Dermatol. 2008; 17:395–404.3. Zhao H, Ozen M, Wong RJ, Stevenson DK. Heme oxygenase-1 in pregnancy and cancer: similarities in cellular invasion, cytoprotection, angiogenesis, and immunomodulation. Front Pharmacol. 2015; 5:295.
Article4. Jian Z, Li K, Liu L, Zhang Y, Zhou Z, Li C, et al. Heme oxygenase-1 protects human melanocytes from H2O2-induced oxidative stress via the Nrf2-ARE pathway. J Invest Dermatol. 2011; 131:1420–1427.
Article5. Jin SA, Park JJ, Lee JB, Lee SC, Yun SJ. Decreased heme oxygenase-1 expression distinguishes human melanomas from melanocytic nevi. Pigment Cell Melanoma Res. 2010; 23:841–844.
Article6. Immenschuh S, Ramadori G. Gene regulation of heme oxygenase-1 as a therapeutic target. Biochem Pharmacol. 2000; 60:1121–1128.
Article7. Box NF, Terzian T. The role of p53 in pigmentation, tanning and melanoma. Pigment Cell Melanoma Res. 2008; 21:525–533.
Article8. Park HY, Kosmadaki M, Yaar M, Gilchrest BA. Cellular mechanisms regulating human melanogenesis. Cell Mol Life Sci. 2009; 66:1493–1506.
Article9. Lee SY, Jo HJ, Kim KM, Song JD, Chung HT, Park YC. Concurrent expression of heme oxygenase-1 and p53 in human retinal pigment epithelial cell line. Biochem Biophys Res Commun. 2008; 365:870–874.
Article10. Nam SY, Sabapathy K. p53 promotes cellular survival in a context-dependent manner by directly inducing the expression of haeme-oxygenase-1. Oncogene. 2011; 30:4476–4486.
Article11. Kim DH, Song NY, Kim EH, Na HK, Joe Y, Chung HT, et al. 15-deoxy-Δ12,14-prostaglandin J2 induces p53 expression through Nrf2-mediated upregulation of heme oxygenase-1 in human breast cancer cells. Free Radic Res. 2014; 48:1018–1027.
Article12. Eisinger M, Lee JS, Hefton JM, Darzynkiewicz Z, Chiao JW, de Harven E. Human epidermal cell cultures: growth and differentiation in the absence of differentiation in the absence of dermal components or medium supplements. Proc Natl Acad Sci U S A. 1979; 76:5340–5344.
Article13. Elassiuty YE, Klarquist J, Speiser J, Yousef RM, El Refaee AA, Hunter NS, et al. Heme oxygenase-1 expression protects melanocytes from stress-induced cell death: implications for vitiligo. Exp Dermatol. 2011; 20:496–501.
Article14. Marrot L, Belaïdi JP, Jones C, Perez P, Meunier JR. Molecular responses to stress induced in normal human caucasian melanocytes in culture by exposure to simulated solar UV. Photochem Photobiol. 2005; 81:367–375.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Translocation of p53 Protein in Melanocytes and Malignant Melanoma Cells After UVB Irradiation
- Heme Oxygenase-1: Its Therapeutic Roles in Inflammatory Diseases
- Activation of Toll-like Receptors 1, 2, 4, 5, and 7 on Human Melanocytes Modulate Pigmentation
- Effects of Oxidative Stress and Antioxidant on the Expression of Heme Oxygenase-1 in Human RPE
- Possible Role of Heme Oxygenase-1 and Prostaglandins in the Pathogenesis of Cerebral Malaria: Heme Oxygenase-1 Induction by Prostaglandin D2 and Metabolite by a Human Astrocyte Cell Line