J Korean Ophthalmol Soc.
2009 Aug;50(8):1247-1253. 10.3341/jkos.2009.50.8.1247.
Effects of Oxidative Stress and Antioxidant on the Expression of Heme Oxygenase-1 in Human RPE
- Affiliations
-
- 1Department of Ophthalmology, Inha University School of Medicine, Incheon, Korea. hschin@inha.ac.kr
- KMID: 2212543
- DOI: http://doi.org/10.3341/jkos.2009.50.8.1247
Abstract
- PURPOSE
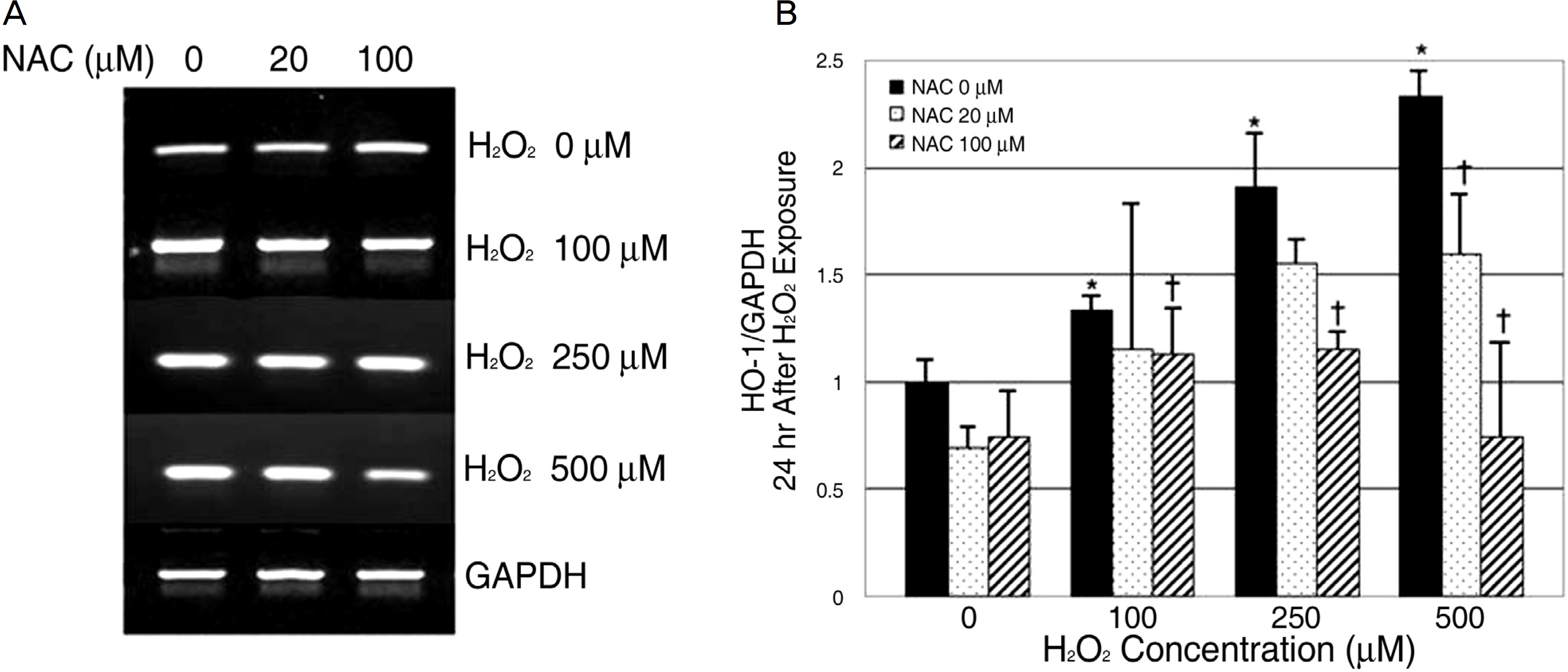
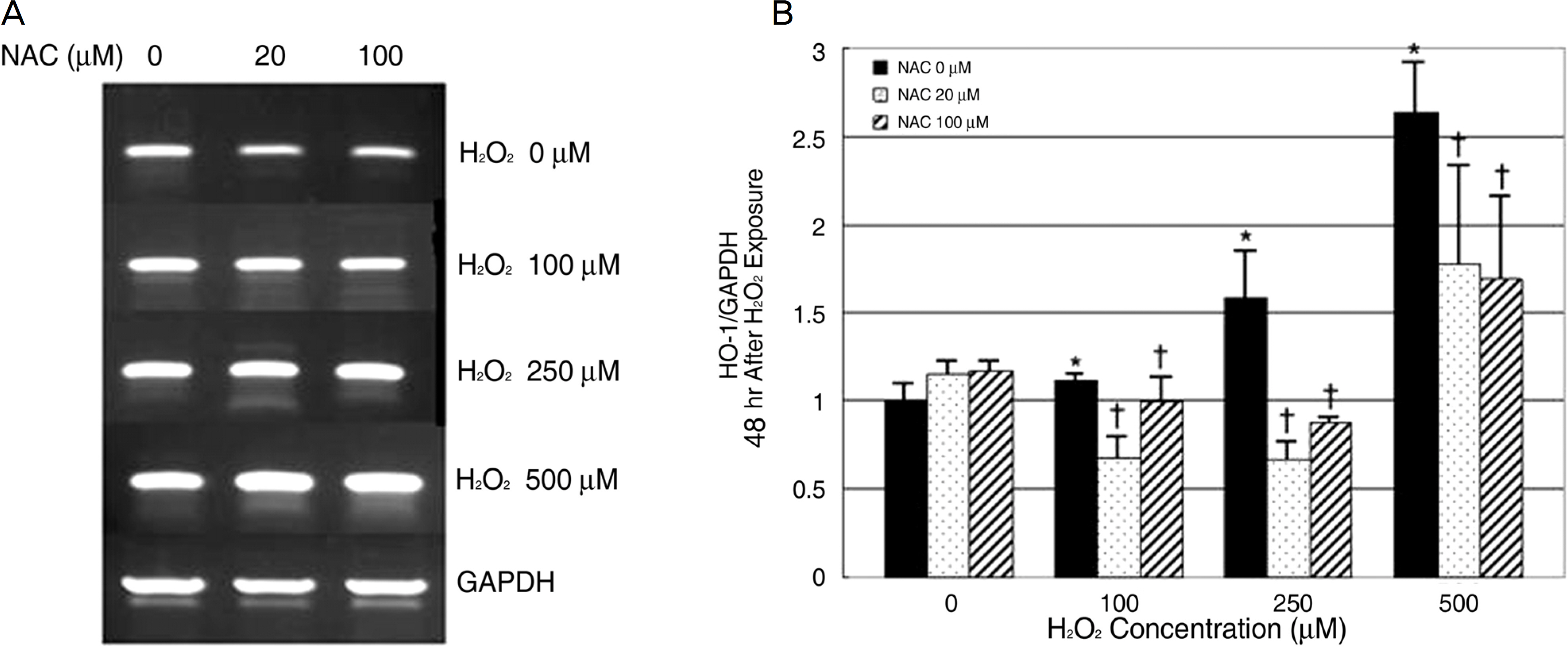
To evaluate the effects of oxidative stress and antioxidantson heme oxygenase-1 (HO-1) in cultured human retinal pigment epithelial (RPE) cells. METHODS: Cultured RPE cells were challenged with different concentrations of hydrogen peroxide (H2O2), and the HO-1 mRNA level was determined by RT-PCR after 24 hours and 48 hours of incubation independently. Additionally, the HO-1 mRNA level was measured after preincubating RPE cells with N-acetylcystein (NAC) as the antioxidant for 30 minutes and then challenging the cells with H2O2. RESULTS: The expression of the HO-1 mRNA level increased after H2O2 exposure, and this level was proportional to the increased H2O2 concentration (p<0.05). Expression of the HO-1 mRNA level decreased when the RPE cells were preincubated with NAC (p<0.05). CONCLUSIONS: In human RPE cells, the HO-1 level increased in proportion to the degree of oxidative stress and decreased with exposure to antioxidants. Expression of HO-1 may be helpful in preventing retinal disease, which occurs due to oxidative stresses such as age-related macular degeneration.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Friedman DS, O'Colmain BJ, Muñoz B, et al. Eye Diseases Prevalence Research Group. Prevalence of Age-Related Macular Degeneration in the United States. Arch Ophthalmol. 2004; 122:564–72.2. Klein R, Wang Q, Klein BE, et al. The relationship of age related maculopathy, cataract, and glaucoma to visual acuity. Invest Ophthalmol Vis Sci. 1995; 36:182–91.3. Williams RA, Brody BL, Thomas RG, et al. The psychosocial impact of macular degeneration. Arch Ophthalmol. 1998; 116:514–20.
Article4. Young RW. Pathophysiology of age-related macular degeneration. Surv Ophthalmol. 1987; 31:291–306.
Article5. Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005; 85:845–81.
Article6. Beatty S, Koh H, Phil M, et al. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000; 45:115–34.
Article7. Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Mol Vis. 1999; 5–32.8. Jaattela M. Heat shock proteins as cellular lifeguards. Ann Med. 1999; 31:261–71.9. David JT, Michael VM, David AN. Phagocytosis and H2 O2 induce catalase and metallothionein gene expression in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1995; 36:1271–9.10. Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci. 1968; 61:748–55.
Article11. Clark JE, Foresti R, Green CJ, Motterlini R. Dynamics of haem oxygenase-1 expression and bilirubin production in cellular protection against oxidative stress. Biochem J. 2000; 348:615–9.
Article12. Stocker R, Yamamoto Y, McDonagh AF, et al. Bilirubin is an antioxidant of possible physiological importance. Science. 1987; 235:1043–6.
Article13. Baranano DE, Wolosker H, Bae BI, et al. A mammalian iron ATPase induced by iron. J Biol Chem. 2000; 275:15166–73.14. Peyton KJ, Reyna SV, Chapman GB, et al. Heme oxygenase-1− derived carbon monoxide is an autocrine inhibitor of vascular smooth muscle cell growth. Blood. 2002; 99:4443–8.15. Otterbein LE, Bach FH, Alam J, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000; 6:422–8.
Article16. Otterbein LE, Zuckerbraun BS, Haga M, et al. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat Med. 2003; 9:183–90.
Article17. Ryter SW, Otterbein LE. Carbon monoxide in biology and medicine. Bioessays. 2004; 26:270–80.
Article18. Maines MD, Trakshel GM, Kutty RK. Characterization of two constitutive forms of rat liver microsomal heme oxygenase: only one molecular species of the enzyme is inducible. J Biol Chem. 1986; 261:411–9.
Article19. Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988; 2:2557–68.
Article20. McCoubrey WK Jr, Huang TJ, Maines MD. Isolation and charac-terization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase− 3. Eur J Biochem. 1997; 247:725–32.21. Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol Rev. 2006; 86:583–650.
Article22. Kutty RK, Nagineni CN, Kutty G, et al. Increased expression of heme oxygenase-1 in human retinal pigment epithelial cells by transforming growth factor-beta. J Cell Physiol. 1994; 159:371–8.23. Kuesap J, Li B, Satarug S, et al. Prostaglandin D2 induces heme oxygenase-1 in human retinal pigment epithelial cells. Biochem Biophys Res Commun. 2008; 367:413–9.
Article24. Udono-Fujimori R, Takahashi K, Takeda K, et al. Expression of heme oxygenase-1 is repressed by interferon-gamma and induced by hypoxia in human retinal pigment epithelial cells. Eur J Biochem. 2004; 271:3076–84.25. Alizadeh M, Wada M, Gelfman CM, et al. Downregulation of Differentiation Specific Gene Expression by Oxidative Stress in ARPE-19 Cells. Invest Ophthalmol Vis Sci. 2001; 42:2706–713.26. McCoubrey WK Jr, Maines MD. The structure, organization and differential expression of the gene encoding rat heme oxygenase-2. Gene. 1994; 139:155–61.
Article27. Hayashi S, Omata Y, Sakamoto H, et al. Characterization of rat heme oxygenase-3 gene: Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene. 2004; 336:241–50.
Article28. Age-Related Eye Disease Study Research Group . A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report No. 8. Arch Ophthalmol. 2001; 119:1417–36.29. Morita T. Heme oxygenase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005; 25:1786–95.
Article30. Hoekstra KA, Godin DV, Cheng KM. Protective role of heme oxygenase in the blood vessel wall during atherogenesis. Biochem Cell Biol. 2004; 82:351–9.
Article31. Nath KA. Heme oxygenase-1: A provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 2006; 70:432–43.
Article32. Donoso LA, Kim D, Frost A, et al. The Role of Inflammation in the Pathogenesis of Age-related Macular Degeneration. Surv Ophthalmol. 2006; 51:137–52.
Article33. Hanneken A, Lin FF, Johnson J, Maher P. Flavonoids Protect Human Retinal Pigment Epithelial Cells from Oxidative-Stress– Induced Death. Invest Ophthalmol Vis Sci. 2006; 47:3164–77.34. Frank RN, Amin RH, Puklin JE. Antioxidant enzymes in the macular retinal pigment epithelium of eyes with neovascular age-related macular degeneration. Am J Ophthalmol. 1999; 127:694–709.35. Milbury PE, Graf B, Curran-Celentano JM, Blumberg JB. Bilberry (Vaccinium myrtillus) anthocyanins modulate heme oxygenase-1 and glutathione S-transferase-pi expression in ARPE− 19 cells. Invest Ophthamol Vis Sci. 2007; 48:2343–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Heme Oxygenase-1: Its Therapeutic Roles in Inflammatory Diseases
- Rosmarinic Acid Attenuates Cell Damage against UVB Radiation-Induced Oxidative Stress via Enhancing Antioxidant Effects in Human HaCaT Cells
- Protopanaxatriol Ginsenoside Rh1 Upregulates Phase II Antioxidant Enzyme Gene Expression in Rat Primary Astrocytes: Involvement of MAP Kinases and Nrf2/ARE Signaling
- A Immunohistochemical Study of Expression of Inducible Isoform of Heme Oxygenase on Human Nasal Polyps
- Evaluation of superoxide dismutase 3, heme-oxygenase, and myeloperoxidase expression levels associated with oxidative stress in chronic periodontitis