Immune Netw.
2009 Feb;9(1):12-19. 10.4110/in.2009.9.1.12.
Heme Oxygenase-1: Its Therapeutic Roles in Inflammatory Diseases
- Affiliations
-
- 1Department of Microbiology and Immunology, Wonkwang University School of Medicine, Iksan, Korea. htchung@wku.ac.kr
- KMID: 2167992
- DOI: http://doi.org/10.4110/in.2009.9.1.12
Abstract
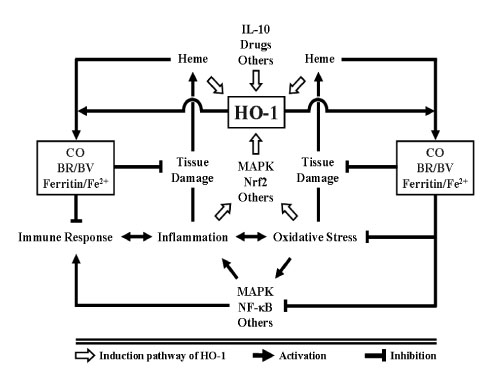
- Heme oxygenase (HO)-1 is an inducible enzyme that catalyzes the first and rate-limiting step in the oxidative degradation of free heme into ferrous iron, carbon monoxide (CO), and biliverdin (BV), the latter being subsequently converted into bilirubin (BR). HO-1, once expressed during inflammation, forms high concentrations of its enzymatic by-products that can influence various biological events, and this expression is proven to be associated with the resolution of inflammation. The degradation of heme by HO-1 itself, the signaling actions of CO, the antioxidant properties of BV/BR, and the sequestration of ferrous iron by ferritin all concertedly contribute to the anti-inflammatory effects of HO-1. This review focuses on the anti-inflammatory mechanisms of HO-1 actions and its roles in inflammatory diseases.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The Stem Bark of Kalopanax pictus Exhibits Anti-inflammatory Effect through Heme Oxygenase-1 Induction and NF-κB Suppression
Soo Young Bang, Ga-Young Park, Sun Young Park, Ji-Hee Kim, Yun Kyoung Lee, Sang-Joon Lee, YoungHee Kim
Immune Netw. 2010;10(6):212-218. doi: 10.4110/in.2010.10.6.212.
Reference
-
1. Kumar S, Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol Lett. 2005. 157:175–188.
Article2. Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med. 2000. 28:289–309.
Article3. Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988. 2:2557–2568.
Article4. Wagener FA, Volk HD, Willis D, Abraham NG, Soares MP, Adema GJ, Figdor CG. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol Rev. 2003. 55:551–571.
Article5. Immenschuh S, Ramadori G. Gene regulation of heme oxygenase-1 as a therapeutic target. Biochem Pharmacol. 2000. 60:1121–1128.
Article6. Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008. 60:79–127.
Article7. Willoughby DA, Moore AR, Colville-Nash PR, Gilroy D. Resolution of inflammation. Int J Immunopharmacol. 2000. 22:1131–1135.
Article8. Owuor ED, Kong AN. Antioxidants and oxidants regulated signal transduction pathways. Biochem Pharmacol. 2002. 64:765–770.
Article9. Farombi EO, Surh YJ. Heme oxygenase-1 as a potential therapeutic target for hepatoprotection. J Biochem Mol Biol. 2006. 39:479–491.
Article10. Ryter SW, Otterbein LE, Morse D, Choi AM. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem. 2002. 234:249–263.
Article11. Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006. 66:11580–11584.
Article12. Kim EH, Na HK, Surh YJ. Upregulation of VEGF by 15-deoxy-Delta12,14-prostaglandin J2 via heme oxygenase-1 and ERK1/2 signaling in MCF-7 cells. Ann N Y Acad Sci. 2006. 1090:375–384.
Article13. Yao P, Nussler A, Liu L, Hao L, Song F, Schirmeier A, Nussler N. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J Hepatol. 2007. 47:253–261.
Article14. Takahashi T, Shimizu H, Morimatsu H, Inoue K, Akagi R, Morita K, Sassa S. Heme oxygenase-1: a fundamental guardian against oxidative tissue injuries in acute inflammation. Mini Rev Med Chem. 2007. 7:745–753.
Article15. Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006. 86:583–650.
Article16. Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000. 6:422–428.
Article17. Pae HO, Oh GS, Choi BM, Chae SC, Kim YM, Chung KR, Chung HT. Carbon monoxide produced by heme oxygenase-1 suppresses T cell proliferation via inhibition of IL-2 production. J Immunol. 2004. 172:4744–4751.
Article18. Suh GY, Jin Y, Yi AK, Wang XM, Choi AM. CCAAT/enhancer-binding protein mediates carbon monoxide-induced suppression of cyclooxygenase-2. Am J Respir Cell Mol Biol. 2006. 35:220–226.
Article19. Megías J, Busserolles J, Alcaraz MJ. The carbon monoxide-releasing molecule CORM-2 inhibits the inflammatory response induced by cytokines in Caco-2 cells. Br J Pharmacol. 2007. 150:977–986.
Article20. Ollinger R, Wang H, Yamashita K, Wegiel B, Thomas M, Margreiter R, Bach FH. Therapeutic applications of bilirubin and biliverdin in transplantation. Antioxid Redox Signal. 2007. 9:2175–2185.
Article21. Yamashita K, McDaid J, Ollinger R, Tsui TY, Berberat PO, Usheva A, Csizmadia E, Smith RN, Soares MP, Bach FH. Biliverdin, a natural product of heme catabolism, induces tolerance to cardiac allografts. FASEB J. 2004. 18:765–767.
Article22. Sarady-Andrews JK, Liu F, Gallo D, Nakao A, Overhaus M, Ollinger R, Choi AM, Otterbein LE. Biliverdin administration protects against endotoxin-induced acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol. 2005. 289:L1131–L1137.
Article23. Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, Yu J, Tsui TY, Bach FH. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol. 2004. 172:3553–3563.
Article24. Balla J, Vercellotti GM, Jeney V, Yachie A, Varga Z, Eaton JW, Balla G. Heme, heme oxygenase and ferritin in vascular endothelial cell injury. Mol Nutr Food Res. 2005. 49:1030–1043.
Article25. Aust SD. Ferritin as a source of iron and protection from iron-induced toxicities. Toxicol Lett. 1995. 82-83:941–944.
Article26. Schaer CA, Schoedon G, Imhof A, Kurrer MO, Schaer DJ. Constitutive endocytosis of CD163 mediates hemoglobin-heme uptake and determines the noninflammatory and protective transcriptional response of macrophages to hemoglobin. Circ Res. 2006. 99:943–950.
Article27. Kapturczak MH, Wasserfall C, Brusko T, Campbell-Thompson M, Ellis TM, Atkinson MA, Agarwal A. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol. 2004. 165:1045–1053.28. Tracz MJ, Juncos JP, Croatt AJ, Ackerman AW, Grande JP, Knutson KL, Kane GC, Terzic A, Griffin MD, Nath KA. Deficiency of heme oxygenase-1 impairs renal hemodynamics and exaggerates systemic inflammatory responses to renal ischemia. Kidney Int. 2007. 72:1073–1080.
Article29. Koizumi S. Human heme oxygenase-1 deficiency: a lesson on serendipity in the discovery of the novel disease. Pediatr Int. 2007. 49:125–132.
Article30. Kawashima A, Oda Y, Yachie A, Koizumi S, Nakanishi I. Heme oxygenase-1 deficiency: the first autopsy case. Hum Pathol. 2002. 33:125–130.
Article31. Baecher-Allan C, Hafler DA. Human regulatory T cells and their role in autoimmune disease. Immunol Rev. 2006. 212:203–216.
Article32. Zelenay S, Chora A, Soares MP, Demengeot J. Heme oxygenase-1 is not required for mouse regulatory T cell development and function. Int Immunol. 2007. 19:11–18.
Article33. George JF, Braun A, Brusko TM, Joseph R, Bolisetty S, Wasserfall CH, Atkinson MA, Agarwal A, Kapturczak MH. Suppression by CD4+CD25+ regulatory T cells is dependent on expression of heme oxygenase-1 in antigen-presenting cells. Am J Pathol. 2008. 173:154–160.
Article34. Maeda S, Nakatsuka I, Hayashi Y, Higuchi H, Shimada M, Miyawaki T. Heme oxygenase-1 induction in the brain during lipopolysaccharide-induced acute inflammation. Neuropsychiatr Dis Treat. 2008. 4:663–667.
Article35. Tamion F, Richard V, Renet S, Thuillez C. Intestinal preconditioning prevents inflammatory response by modulating heme oxygenase-1 expression in endotoxic shock model. Am J Physiol Gastrointest Liver Physiol. 2007. 293:G1308–G1314.
Article36. Tracz MJ, Juncos JP, Grande JP, Croatt AJ, Ackerman AW, Rajagopalan G, Knutson KL, Badley AD, Griffin MD, Alam J, Nath KA. Renal hemodynamic, inflammatory, and apoptotic responses to lipopolysaccharide in HO-1-/- mice. Am J Pathol. 2007. 170:1820–1830.
Article37. Tamion F, Richard V, Renet S, Thuillez C. Protective effects of heme-oxygenase expression against endotoxic shock: inhibition of tumor necrosis factor-alpha and augmentation of interleukin-10. J Trauma. 2006. 61:1078–1084.
Article38. Moreto V, Stabile AM, Antunes-Rodrigues J, Carnio EC. Role of heme-oxygenase pathway on vasopressin deficiency during endotoxemic shock-like conditions. Shock. 2006. 26:472–476.
Article39. Chang KY, Tsai PS, Huang TY, Wang TY, Yang S, Huang CJ. HO-1 mediates the effects of HBO pretreatment against sepsis. J Surg Res. 2006. 136:143–153.
Article40. Poole B, Wang W, Chen YC, Zolty E, Falk S, Mitra A, Schrier R. Role of heme oxygenase-1 in endotoxemic acute renal failure. Am J Physiol Renal Physiol. 2005. 289:F1382–F1385.
Article41. Wiesel P, Patel AP, DiFonzo N, Marria PB, Sim CU, Pellacani A, Maemura K, LeBlanc BW, Marino K, Doerschuk CM, Yet SF, Lee ME, Perrella MA. Endotoxin-induced mortality is related to increased oxidative stress and end-organ dysfunction, not refractory hypotension, in heme oxygenase-1-deficient mice. Circulation. 2000. 102:3015–3022.
Article42. Suzuki T, Takahashi T, Yamasaki A, Fujiwara T, Hirakawa M, Akagi R. Tissue-specific gene expression of heme oxygenase-1 (HO-1) and non-specific delta-aminolevulinate synthase (ALAS-N) in a rat model of septic multiple organ dysfunction syndrome. Biochem Pharmacol. 2000. 60:275–283.
Article43. Mohri T, Ogura H, Koh T, Fujita K, Sumi Y, Yoshiya K, Matsushima A, Hosotsubo H, Kuwagata Y, Tanaka H, Shimazu T, Sugimoto H. Enhanced expression of intracellular heme oxygenase-1 in deactivated monocytes from patients with severe systemic inflammatory response syndrome. J Trauma. 2006. 61:616–623.
Article44. Chung SW, Liu X, Macias AA, Baron RM, Perrella MA. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J Clin Invest. 2008. 118:239–247.
Article45. Becker T, Zu Vilsendorf AM, Terbish T, Klempnauer J, Jörns A. Induction of heme oxygenase-1 improves the survival of pancreas grafts by prevention of pancreatitis after transplantation. Transplantation. 2007. 84:1644–1655.
Article46. Du D, Chang S, Chen B, Zhou H, Chen ZK. Adenovirus-mediated heme oxygenase transfer inhibits graft arteriosclerosis in rat aortic transplants. Transplant Proc. 2007. 39:3446–3448.
Article47. Laumonier T, Yang S, Konig S, Chauveau C, Anegon I, Hoffmeyer P, Menetrey J. Lentivirus mediated HO-1 gene transfer enhances myogenic precursor cell survival after autologous transplantation in pig. Mol Ther. 2008. 16:404–410.
Article48. Zhen-Wei X, Jian-Le S, Qi Q, Wen-Wei Z, Xue-Hong Z, Zi-Li Z. Heme oxygenase-1 improves the survival of discordant cardiac xenograft through its anti-inflammatory and anti-apoptotic effects. Pediatr Transplant. 2007. 11:850–859.
Article49. Lee SS, Gao W, Mazzola S, Thomas MN, Csizmadia E, Otterbein LE, Bach FH, Wang H. Heme oxygenase-1, carbon monoxide, and bilirubin induce tolerance in recipients toward islet allografts by modulating T regulatory cells. FASEB J. 2007. 21:3450–3457.
Article50. Schnickel GT, Hsieh GR, Kachikwu EL, Garcia C, Shefizadeh A, Fishbein MC, Ardehali A. Cytoprotective gene HO-1 and chronic rejection in heart transplantation. Transplant Proc. 2006. 38:3259–3262.
Article51. Yamashita K, Ollinger R, McDaid J, Sakahama H, Wang H, Tyagi S, Csizmadia E, Smith NR, Soares MP, Bach FH. Heme oxygenase-1 is essential for and promotes tolerance to transplanted organs. FASEB J. 2006. 20:776–778.
Article52. Tu CF, Kuo CH, Juang JH. Effects of heme oxygenase-1 transgenic islets on transplantation. Transplant Proc. 2005. 37:3463–3467.
Article53. Wang H, Lee SS, Gao W, Czismadia E, McDaid J, Ollinger R, Soares MP, Yamashita K, Bach FH. Donor treatment with carbon monoxide can yield islet allograft survival and tolerance. Diabetes. 2005. 54:1400–1406.
Article54. Kobayashi T, Sato Y, Yamamoto S, Takeishi T, Hirano K, Watanabe T, Takano K, Naito M, Hatakeyama K. Augmentation of heme oxygenase-1 expression in the graft immediately after implantation in adult living-donor liver transplantation. Transplantation. 2005. 79:977–980.
Article55. Martins PN, Kessler H, Jurisch A, Reutzel-Selke A, Kramer J, Pascher A, Pratschke J, Neuhaus P, Volk HD, Tullius SG. Induction of heme oxygenase-1 in the donor reduces graft immunogenicity. Transplant Proc. 2005. 37:384–386.
Article56. Martins PN, Reuzel-Selke A, Jurisch A, Atrott K, Pascher A, Pratschke J, Buelow R, Neuhaus P, Volk HD, Tullius SG. Induction of carbon monoxide in the donor reduces graft immunogenicity and chronic graft deterioration. Transplant Proc. 2005. 37:379–381.
Article57. Bédard EL, Jiang J, Parry N, Wang H, Liu W, Garcia B, Kim P, Chakrabarti S, Buelow R, Zhong R. Peritransplant treatment with cobalt protoporphyrin attenuates chronic renal allograft rejection. Transpl Int. 2005. 18:341–349.
Article58. Sato K, Balla J, Otterbein L, Smith RN, Brouard S, Lin Y, Csizmadia E, Sevigny J, Robson SC, Vercellotti G, Choi AM, Bach FH, Soares MP. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J Immunol. 2001. 166:4185–4194.
Article59. Nakao A, Choi AM, Murase N. Protective effect of carbon monoxide in transplantation. J Cell Mol Med. 2006. 10:650–671.
Article60. Wagner M, Cadetg P, Ruf R, Mazzucchelli L, Ferrari P, Redaelli CA. Heme oxygenase-1 attenuates ischemia/reperfusion-induced apoptosis and improves survival in rat renal allografts. Kidney Int. 2003. 63:1564–1573.
Article61. Bach FH. Heme oxygenase-1 and transplantation tolerance. Hum Immunol. 2006. 67:430–432.
Article62. Shen WL, Zhong MF, Ding WL, Wang J, Zheng L, Zhu P, Wang BS, Higashino H, Chen H. Elevated catalase and heme oxygenase-1 may contribute to improved postischaemic cardiac function in long-term type 1 diabetes. Clin Exp Pharmacol Physiol. 2008. 35:820–826.
Article63. Jang JU, Lee SH, Choi CU, Bahk SC, Chung HT, Yang YS. Effects of heme oxygenase-1 inducer and inhibitor on experimental autoimmune uveoretinitis. Korean J Ophthalmol. 2007. 21:238–243.
Article64. Hu CM, Lin HH, Chiang MT, Chang PF, Chau LY. Systemic expression of heme oxygenase-1 ameliorates type 1 diabetes in NOD mice. Diabetes. 2007. 56:1240–1247.
Article65. Li M, Peterson S, Husney D, Inaba M, Guo K, Kappas A, Ikehara S, Abraham NG. Long-lasting expression of HO-1 delays progression of type I diabetes in NOD mice. Cell Cycle. 2007. 6:567–571.
Article66. Chora AA, Fontoura P, Cunha A, Pais TF, Cardoso S, Ho PP, Lee LY, Sobel RA, Steinman L, Soares MP. Heme oxygenase-1 and carbon monoxide suppress autoimmune neuroinflammation. J Clin Invest. 2007. 117:438–447.
Article67. Kobayashi H, Takeno M, Saito T, Takeda Y, Kirino Y, Noyori K, Hayashi T, Ueda A, Ishigatsubo Y. Regulatory role of heme oxygenase 1 in inflammation of rheumatoid arthritis. Arthritis Rheum. 2006. 54:1132–1142.
Article68. Chakrabarty A, Emerson MR, LeVine SM. Heme oxygenase-1 in SJL mice with experimental allergic encephalomyelitis. Mult Scler. 2003. 9:372–381.
Article69. Liu Y, Zhu B, Luo L, Li P, Paty DW, Cynader MS. Heme oxygenase-1 plays an important protective role in experimental autoimmune encephalomyelitis. Neuroreport. 2001. 12:1841–1845.
Article70. Li M, Peterson S, Husney D, Inaba M, Guo K, Terada E, Morita T, Patil K, Kappas A, Ikehara S, Abraham NG. Interdiction of the diabetic state in NOD mice by sustained induction of heme oxygenase: possible role of carbon monoxide and bilirubin. Antioxid Redox Signal. 2007. 9:855–863.
Article71. Pae HO, Lee YC, Chung HT. Heme oxygenase-1 and carbon monoxide: emerging therapeutic targets in inflammation and allergy. Recent Pat Inflamm Allergy Drug Discov. 2008. 2:159–165.
Article72. Almolki A, Taillé C, Martin GF, Jose PJ, Zedda C, Conti M, Megret J, Henin D, Aubier M, Boczkowski J. Heme oxygenase attenuates allergen-induced airway inflammation and hyperreactivity in guinea pigs. Am J Physiol Lung Cell Mol Physiol. 2004. 287:L26–L34.
Article73. Kirino M, Kirino Y, Takeno M, Nagashima Y, Takahashi K, Kobayashi M, Murakami S, Hirasawa T, Ueda A, Aihara M, Ikezawa Z, Ishigatsubo Y. Heme oxygenase 1 attenuates the development of atopic dermatitis-like lesions in mice: implications for human disease. J Allergy Clin Immunol. 2008. 122:290–297.
Article74. Takamiya R, Murakami M, Kajimura M, Goda N, Makino N, Takamiya Y, Yamaguchi T, Ishimura Y, Hozumi N, Suematsu M. Stabilization of mast cells by heme oxygenase-1: an anti-inflammatory role. Am J Physiol Heart Circ Physiol. 2002. 283:H861–H870.75. Vannacci A, Baronti R, Zagli G, Marzocca C, Pierpaoli S, Bani D, Passani MB, Mannaioni PF, Masini E. Carbon monoxide modulates the response of human basophils to FcepsilonRI stimulation through the heme oxygenase pathway. Eur J Pharmacol. 2003. 465:289–297.
Article76. Xia ZW, Xu LQ, Zhong WW, Wei JJ, Li NL, Shao J, Li YZ, Yu SC, Zhang ZL. Heme oxygenase-1 attenuates ovalbumin-induced airway inflammation by up-egulation of foxp3 T-regulatory cells, interleukin-10, and membrane-bound transforming growth factor-1. Am J Pathol. 2007. 171:1904–1914.
Article77. Rushworth SA, O'Connell MA. Haem oxygenase-1 in inflammation. Biochem Soc Trans. 2004. 32:1093–1094.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Carbon Monoxide in Neurovascular Repair Processing
- Carbon monoxide: present and future indications for a medical gas
- Possible Role of Heme Oxygenase-1 and Prostaglandins in the Pathogenesis of Cerebral Malaria: Heme Oxygenase-1 Induction by Prostaglandin D2 and Metabolite by a Human Astrocyte Cell Line
- Low Dose Carbon Monoxide Inhalation Prevents Chronic Allograft Nephropathy following Kidney Transplantation in Rats. Heme Oxygenase-1 Derivatives Study I
- Effects of Oxidative Stress and Antioxidant on the Expression of Heme Oxygenase-1 in Human RPE