Ann Dermatol.
2010 Nov;22(4):486-489. 10.5021/ad.2010.22.4.486.
Activation of Toll-like Receptors 1, 2, 4, 5, and 7 on Human Melanocytes Modulate Pigmentation
- Affiliations
-
- 1Department of Dermatology, Center for Cell Death Regulating Biodrugs, Ajou University School of Medicine, Suwon, Korea. hykang@ajou.ac.kr
- KMID: 2266195
- DOI: http://doi.org/10.5021/ad.2010.22.4.486
Abstract
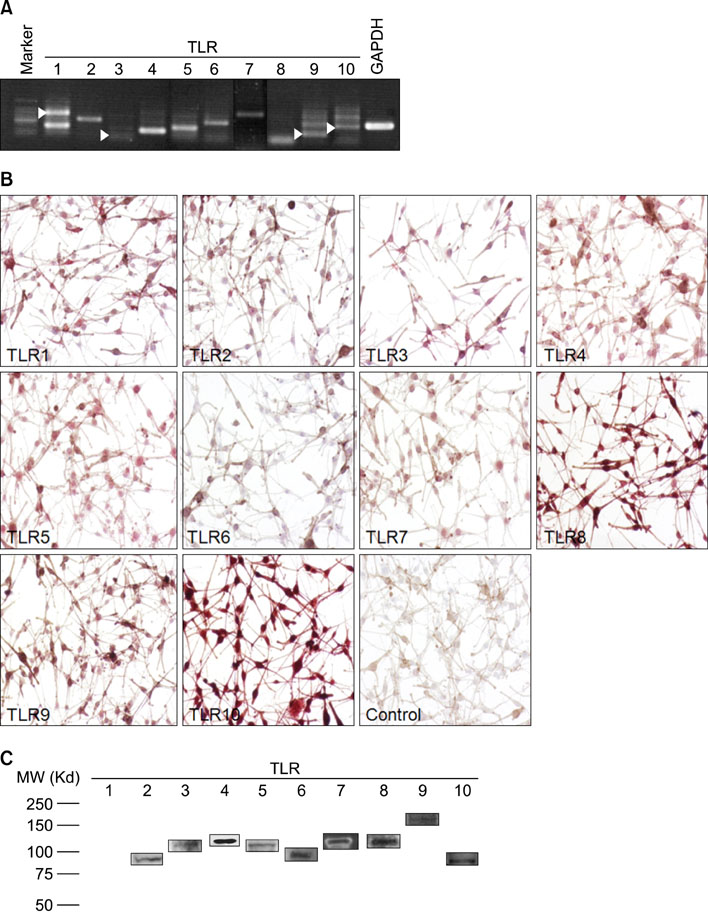
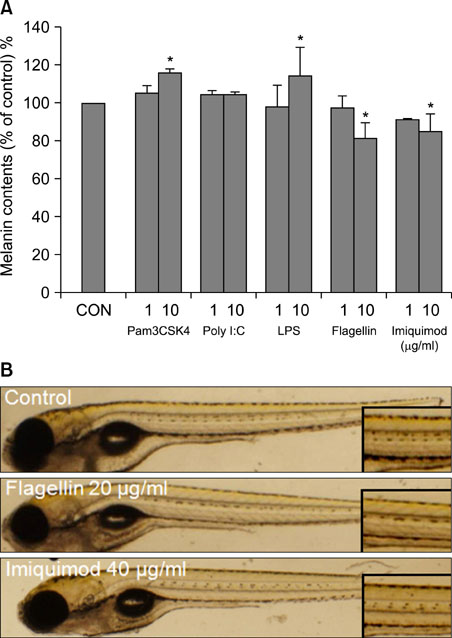
- Human melanocytes are not simply pigment-producing cells. It may be part of the inflammatory response, during which the pigmentary system may produce more melanin or suppress melanization. Toll-like receptors (TLRs) have been implicated in both innate host defense against pathogens and inflammatory response. Therefore, it may be possible that activation of TLRs in melanocytes may play a role in the modulation of melanogenesis. In this study, we investigated whether normal human melanocytes expressed TLRs and analyzed pigmentation changes upon TLR stimulation. The expression of TLR1~10 mRNA in cultured human melanocyte was analyzed using RT-PCR, Western blotting and immunocytochemistry. Human melanocytes constitutively express mRNA and protein for TLR2, 3, 4, 5, 7, 9 and 10. Stimulation of TLR1/2 and 4 with Pam3CSK4 and lipopolysaccharide induced pigmentation of melanocytes. Activation of TLR5 and 7 with flagellin and imiquimod treatments reduced pigmentation of melanocytes and zebrafish. In summary, the results provided evidence for TLRs expression in normal human melanocytes. It is speculated that a response of melanocyte to TLR ligands may play a role in the pigmentary change in the skin.
Keyword
MeSH Terms
Figure
Reference
-
1. Plonka PM, Passeron T, Brenner M, Tobin DJ, Shibahara S, Thomas A, et al. What are melanocytes really doing all day long...? Exp Dermatol. 2009. 18:799–819.2. Terhorst D, Kalali BN, Ollert M, Ring J, Mempel M. The role of toll-like receptors in host defenses and their relevance to dermatologic diseases. Am J Clin Dermatol. 2010. 11:1–10.
Article3. Mackintosh JA. The antimicrobial properties of melanocytes, melanosomes and melanin and the evolution of black skin. J Theor Biol. 2001. 211:101–113.
Article4. Yu N, Zhang S, Zuo F, Kang K, Guan M, Xiang L. Cultured human melanocytes express functional toll-like receptors 2-4, 7 and 9. J Dermatol Sci. 2009. 56:113–120.
Article5. Ahn JH, Park TJ, Jin SH, Kang HY. Human melanocytes express functional Toll-like receptor 4. Exp Dermatol. 2008. 17:412–417.
Article6. Kang HY, Park TJ, Jin SH. Imiquimod, a Toll-like receptor 7 agonist, inhibits melanogenesis and proliferation of human melanocytes. J Invest Dermatol. 2009. 129:243–246.
Article7. Choi TY, Kim JH, Ko DH, Kim CH, Hwang JS, Ahn S, et al. Zebrafish as a new model for phenotype-based screening of melanogenic regulatory compounds. Pigment Cell Res. 2007. 20:120–127.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Donor Specific Response of Estrogen and Progesterone on Cultured Human Melanocytes
- Use of Human Skin Reconstructs Containing Melanocytes as a Model to Evaluate the Effect of Pigment Modifiers
- The Effect of Supernatant from UVB - Irradiated Cultured Keratinocytes on the Growth , Melanin Content , and Tyrosinase Activity of Human Melanocyte
- The Gate Way of Communication between Microorganism and Human Body: Toll-like Receptors
- Do Toll-like Receptors Play a New Role as a Biomarker of Irritable Bowel Syndrome?