Clin Exp Otorhinolaryngol.
2012 Sep;5(3):161-169. 10.3342/ceo.2012.5.3.161.
Phorbol 12-Myristate 13-Acetate Induces MUC16 Expression via PKCdelta and p38 in Human Airway Epithelial Cells
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Yeungnam University College of Medicine, Daegu, Korea. ydkim@med.yu.ac.kr
- 2Department of Otorhinolaryngology, Daegu Fatima Hospital, Daegu, Korea.
- 3Center for Respiratory Disease, Yeungnam University Medical Center, Daegu, Korea.
- KMID: 2134600
- DOI: http://doi.org/10.3342/ceo.2012.5.3.161
Abstract
OBJECTIVES
Phorbol 12-myristate 13-acetate (PMA) is widely used as a protein kinase C (PKC) activator, PKC is involved in the secretion of mucins. MUC16, one of the membrane-bound mucins, is produced in human airway epithelial cells. However, the effect and signaling pathway of PMA on MUC16 expression in human airway epithelial cells has not been reported. Therefore, the effect and brief signaling pathway of PMA on MUC16 expression were investigated in human airway epithelial cells in this study.
METHODS
In the mucin-producing human NCI-H292 airway epithelial cells and the primary cultures of normal nasal epithelial cells, the effect and signaling pathway of PMA on MUC16 expression were investigated using reverse transcriptase-polymerase chain reaction (RT-PCR), real-time PCR, enzyme immunoassay, and immunoblot analysis with several specific inhibitors and small interfering RNA (siRNA) for p38 mitogen-activated protein kinase (MAPK).
RESULTS
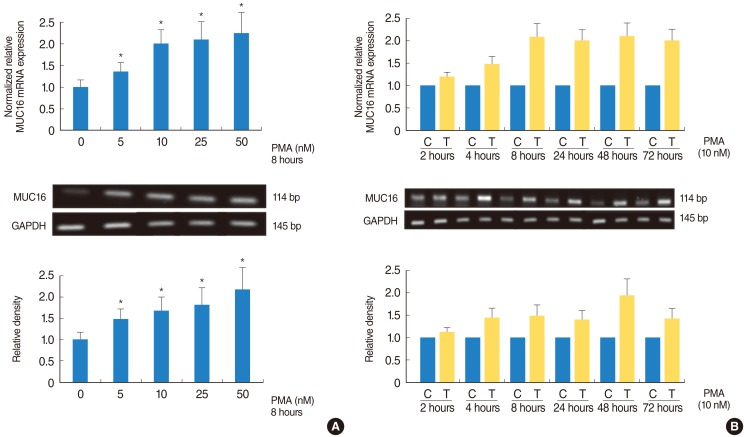
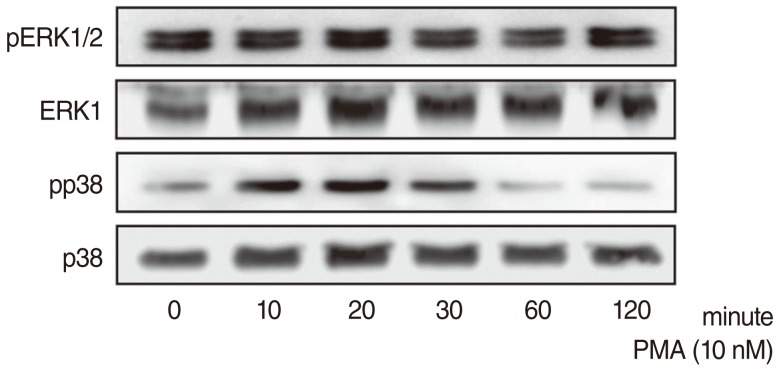
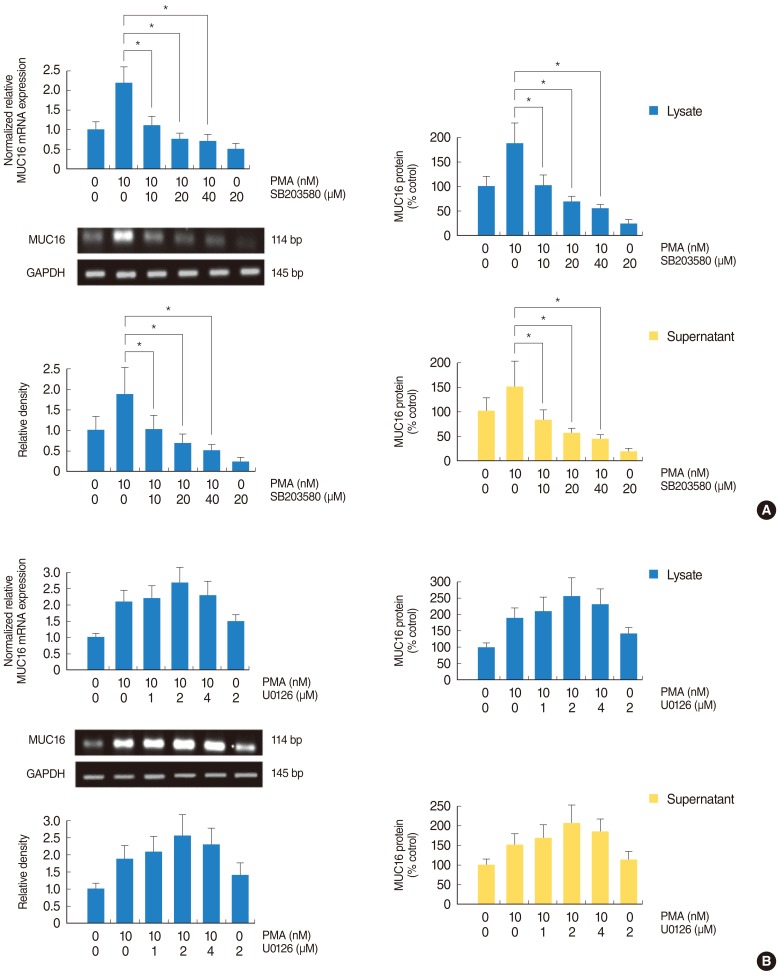
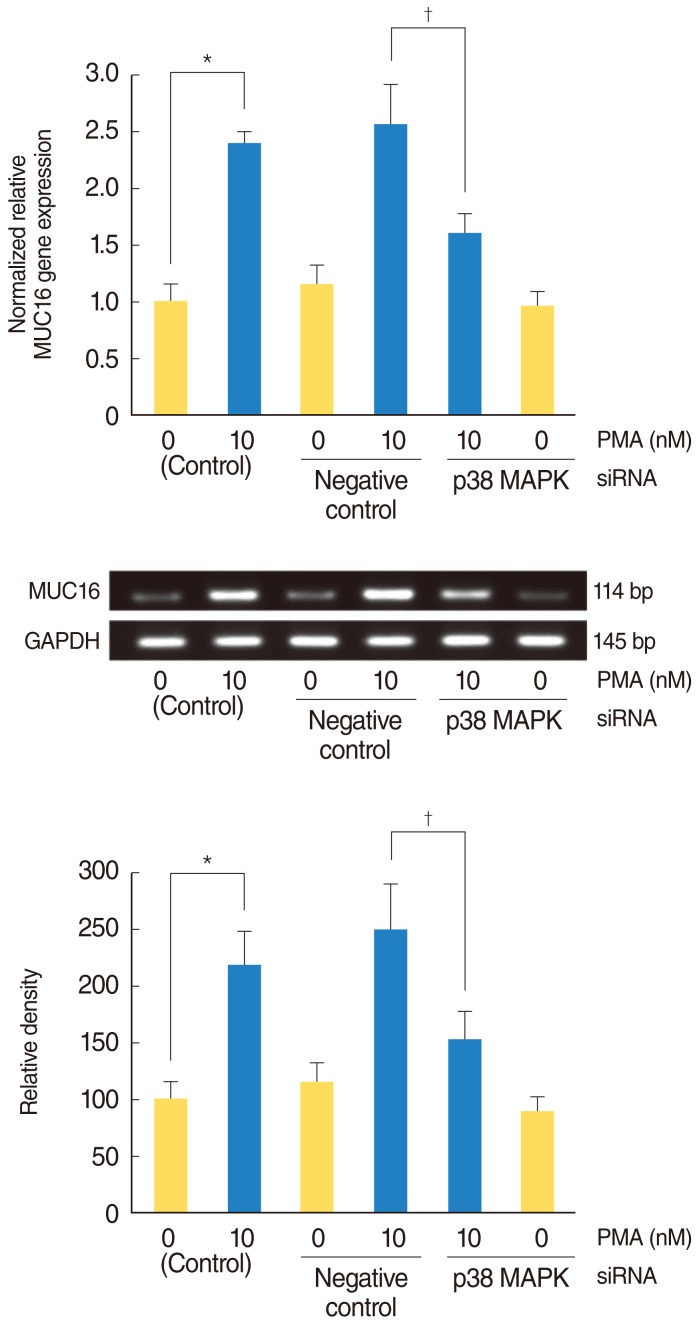
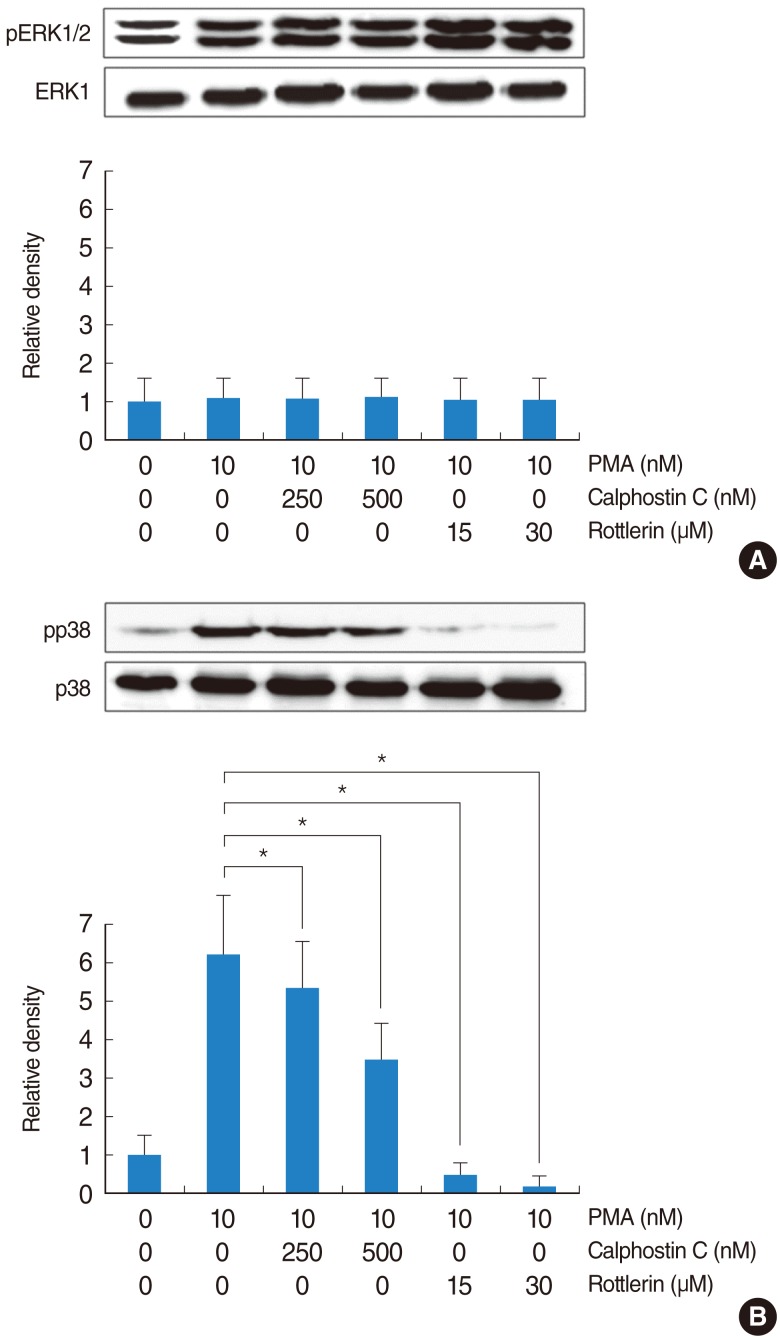
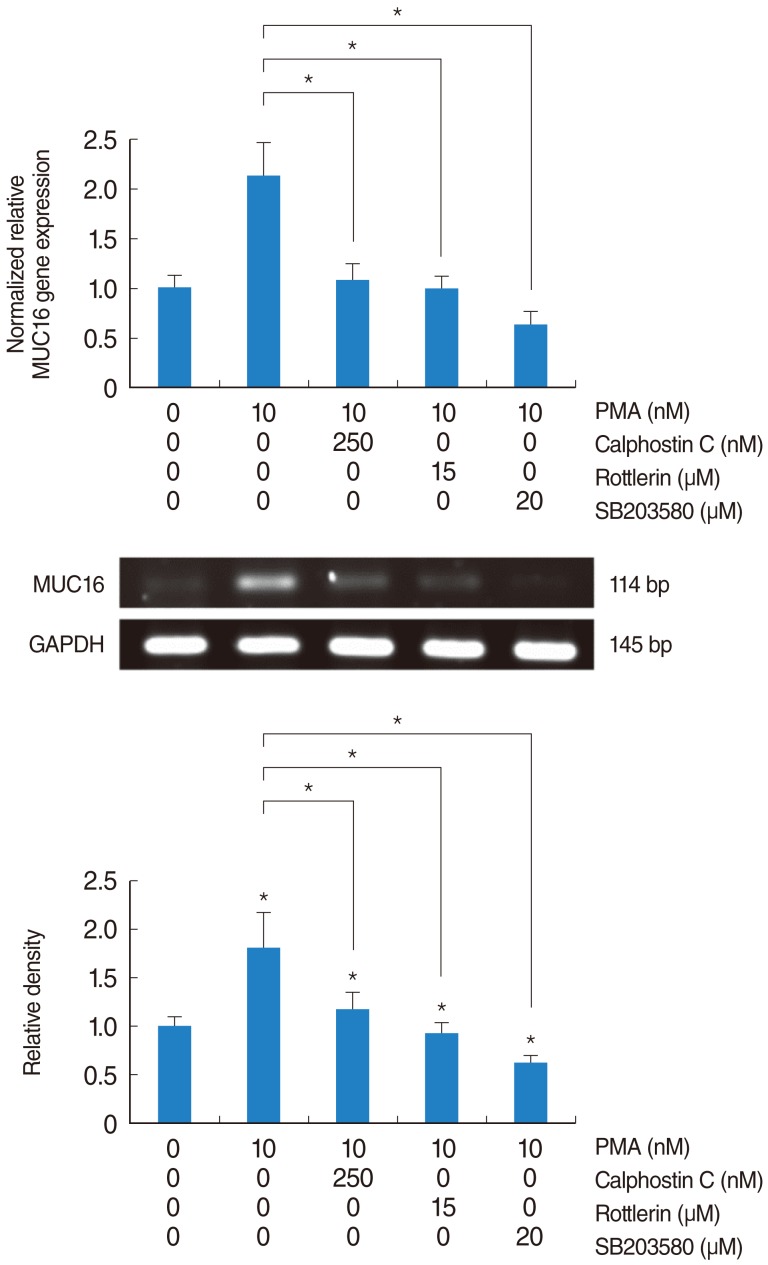
PMA increased MUC16 expression, and activated phosphorylation of p38 MAPK. However, it did not activate phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2). SB203580 (p38 MAPK inhibitor) inhibited PMA-induced MUC16 expression, while U0126 (ERK1/2 inhibitor) did not. In addition, the knockdown of p38 MAPK by p38 MAPK siRNA significantly blocked PMA-induced MUC16 mRNA expression. Rottlerin (PKCdelta inhibitor) inhibited PMA-induced MUC16 expression, and also inhibited the phosphorylation of activated p38 MAPK by PMA.
CONCLUSION
These results show for the first time that PMA-induced MUC16 expression is regulated by activation of the PKCdelta and p38 MAPK signaling pathway in human airway epithelial cells.
MeSH Terms
-
Acetophenones
Benzopyrans
Butadienes
Epithelial Cells
Humans
Imidazoles
Immunoenzyme Techniques
Mucins
Nitriles
p38 Mitogen-Activated Protein Kinases
Phorbols
Phosphorylation
Phosphotransferases
Protein Kinase C
Protein Kinases
Pyridines
Real-Time Polymerase Chain Reaction
RNA, Messenger
RNA, Small Interfering
Acetophenones
Benzopyrans
Butadienes
Imidazoles
Mucins
Nitriles
Phorbols
Phosphotransferases
Protein Kinase C
Protein Kinases
Pyridines
RNA, Messenger
RNA, Small Interfering
p38 Mitogen-Activated Protein Kinases
Figure
Cited by 1 articles
-
Asian Sand Dust Up-Regulates MUC4 Expression in Human Upper Airway Epithelial Cells
Chang-Hwi Park, Yoo Sun Song, Chang Hoon Bae, Yoon Seok Choi, Si-Youn Song, Kyeong-Cheol Shin, Hyun Jung Jin, Yong-Dae Kim
Korean J Otorhinolaryngol-Head Neck Surg. 2017;60(5):222-231. doi: 10.3342/kjorl-hns.2016.17594.
Reference
-
1. Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006; 1. 86(1):245–278. PMID: 16371599.
Article2. Ali MS, Pearson JP. Upper airway mucin gene expression: a review. Laryngoscope. 2007; 5. 117(5):932–938. PMID: 17473699.
Article3. Kim YD, Kwon EJ, Park DW, Song SY, Yoon SK, Baek SH. Interleukin-1beta induces MUC2 and MUC5AC synthesis through cyclooxygenase-2 in NCI-H292 cells. Mol Pharmacol. 2002; 11. 62(5):1112–1118. PMID: 12391274.4. Woo HJ, Bae CH, Song SY, Lee HM, Kim YD. Expression of membrane-bound mucins in human nasal mucosa: different patterns for MUC4 and MUC16. Arch Otolaryngol Head Neck Surg. 2010; 6. 136(6):603–609. PMID: 20566912.5. Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008; 70:431–457. PMID: 17850209.
Article6. Woo HJ, Yoo WJ, Bae CH, Song SY, Kim YW, Park SY, et al. Leptin up-regulates MUC5B expression in human airway epithelial cells via mitogen-activated protein kinase pathway. Exp Lung Res. 2010; 6. 36(5):262–269. PMID: 20497020.
Article7. Blalock TD, Spurr-Michaud SJ, Tisdale AS, Heimer SR, Gilmore MS, Ramesh V, et al. Functions of MUC16 in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2007; 10. 48(10):4509–4518. PMID: 17898272.
Article8. Davies JR, Kirkham S, Svitacheva N, Thornton DJ, Carlstedt I. MUC16 is produced in tracheal surface epithelium and submucosal glands and is present in secretions from normal human airway and cultured bronchial epithelial cells. Int J Biochem Cell Biol. 2007; 39(10):1943–1954. PMID: 17604678.
Article9. Higuchi T, Orita T, Katsuya K, Yamasaki Y, Akiyama K, Li H, et al. MUC20 suppresses the hepatocyte growth factor-induced Grb2-Ras pathway by binding to a multifunctional docking site of met. Mol Cell Biol. 2004; 9. 24(17):7456–7468. PMID: 15314156.
Article10. Gipson IK. Human endocervical mucins. Ernst Schering Res Found Workshop. 2005; (52):219–244. PMID: 15704474.
Article11. Desseyn JL, Tetaert D, Gouyer V. Architecture of the large membrane-bound mucins. Gene. 2008; 3. 410(2):215–222. PMID: 18242885.
Article12. Nustad K, Lebedin Y, Lloyd KO, Shigemasa K, de Bruijn HW, Jansson B, et al. Epitopes on CA 125 from cervical mucus and ascites fluid and characterization of six new antibodies: third report from the ISOBM TD-1 workshop. Tumour Biol. 2002; Sep-Oct. 23(5):303–314. PMID: 12595747.13. Argueso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest Ophthalmol Vis Sci. 2003; 6. 44(6):2487–2495. PMID: 12766047.14. Perez BH, Gipson IK. Focus on molecules: human mucin MUC16. Exp Eye Res. 2008; 11. 87(5):400–401. PMID: 18289532.
Article15. Kesimer M, Scull M, Brighton B, DeMaria G, Burns K, O'Neal W, et al. Characterization of exosome-like vesicles released from human tracheobronchial ciliated epithelium: a possible role in innate defense. FASEB J. 2009; 6. 23(6):1858–1868. PMID: 19190083.
Article16. Albertsmeyer AC, Kakkassery V, Spurr-Michaud S, Beeks O, Gipson IK. Effect of pro-inflammatory mediators on membrane-associated mucins expressed by human ocular surface epithelial cells. Exp Eye Res. 2010; 3. 90(3):444–451. PMID: 20036239.
Article17. Govindarajan B, Gipson IK. Membrane-tethered mucins have multiple functions on the ocular surface. Exp Eye Res. 2010; 6. 90(6):655–663. PMID: 20223235.
Article18. Hewson CA, Edbrooke MR, Johnston SL. PMA induces the MUC5AC respiratory mucin in human bronchial epithelial cells, via PKC, EGF/TGF-alpha, Ras/Raf, MEK, ERK and Sp1-dependent mechanisms. J Mol Biol. 2004; 11. 344(3):683–695. PMID: 15533438.19. Park JA, Crews AL, Lampe WR, Fang S, Park J, Adler KB. Protein kinase C delta regulates airway mucin secretion via phosphorylation of MARCKS protein. Am J Pathol. 2007; 12. 171(6):1822–1830. PMID: 18055557.20. Lee HW, Ahn DH, Crawley SC, Li JD, Gum JR Jr, Basbaum CB, et al. Phorbol 12-myristate 13-acetate up-regulates the transcription of MUC2 intestinal mucin via Ras, ERK, and NF-kappa B. J Biol Chem. 2002; 9. 277(36):32624–32631. PMID: 12077118.21. Wu DY, Wu R, Chen Y, Tarasova N, Chang MM. PMA stimulates MUC5B gene expression through an Sp1-based mechanism in airway epithelial cells. Am J Respir Cell Mol Biol. 2007; 11. 37(5):589–597. PMID: 17600309.22. Yuan-Chen Wu D, Wu R, Reddy SP, Lee YC, Chang MM. Distinctive epidermal growth factor receptor/extracellular regulated kinase-independent and -dependent signaling pathways in the induction of airway mucin 5B and mucin 5AC expression by phorbol 12-myristate 13-acetate. Am J Pathol. 2007; 1. 170(1):20–32. PMID: 17200179.
Article23. Park JA, He F, Martin LD, Li Y, Chorley BN, Adler KB. Human neutrophil elastase induces hypersecretion of mucin from well-differentiated human bronchial epithelial cells in vitro via a protein kinase C{delta}-mediated mechanism. Am J Pathol. 2005; 9. 167(3):651–661. PMID: 16127146.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Eupatilin downregulates phorbol 12-myristate 13-acetate-induced MUC5AC expression via inhibition of p38/ERK/JNK MAPKs signal pathway in human airway epithelial cells
- The Effect of Doxycycline on PMA-Induced MUC5B Expression via MMP-9 and p38 in NCI-H292 Cells
- Galangin Regulates Mucin 5AC Gene Expression via the Nuclear Factor-κB Inhibitor α/Nuclear Factor-κB p65 Pathway in Human Airway Epithelial Cells
- Regulation of fibronectin gene expression by cyclic AMP and phorbol myristate acetate in HT-1080 human fibrosarcoma cells
- Effect of Epigallocatechin-3-Gallate on PMA-Induced MUC5B Expression in Human Airway Epithelial Cells