Clin Exp Otorhinolaryngol.
2013 Dec;6(4):237-242.
Effect of Epigallocatechin-3-Gallate on PMA-Induced MUC5B Expression in Human Airway Epithelial Cells
- Affiliations
-
- 1Department of Physiology, Yeungnam University College of Medicine, Daegu, Korea.
- 2Department of Otorhinolaryngology-Head and Neck Surgery, Yeungnam University College of Medicine, Daegu, Korea. ydkim@med.yu.ac.kr
- 3Regional Center for Respiratory Diseases, Yeungnam University Medical Center, Daegu, Korea.
Abstract
OBJECTIVES
Among the inflammatory mediators, phorbol 12-myristate 13-acetate (PMA) is associated with the regulation of MUC5B expression in the airway epithelial cells. Epigallocatechin-3-gallate (EGCG) is the major component of green tea extract. The biological activity of EGCG includes reduction of cholesterol and antioxidant activity, as well as anti-inflammatory effect. However, the precise action mechanism of anti-inflammatory effect of EGCG in the airway epithelial cells has not been fully defined. This study investigates the effect and the brief signaling pathway of EGCG on PMA-induced MUC5B expression in the airway epithelial cells.
METHODS
In NCI-H292 airway epithelial cells, the effect and signaling pathway of EGCG on MUC5B expression were investigated using real-time polymerase chain reaction analysis, enzyme immunoassay, immunohistochemical analysis, gelatin zymography assay, and immunoblot analysis.
RESULTS
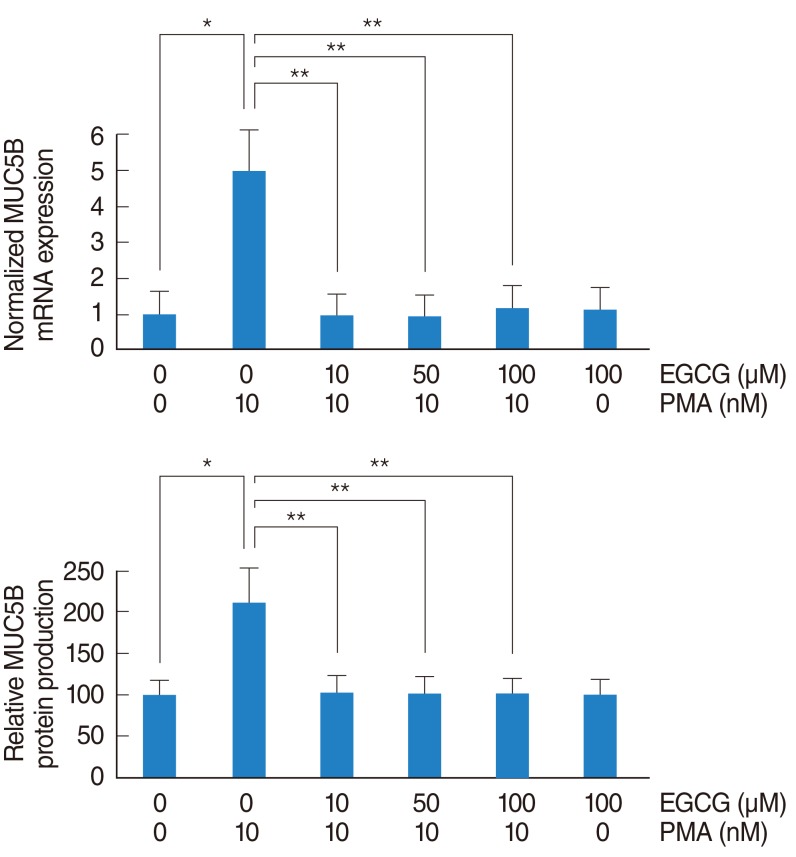
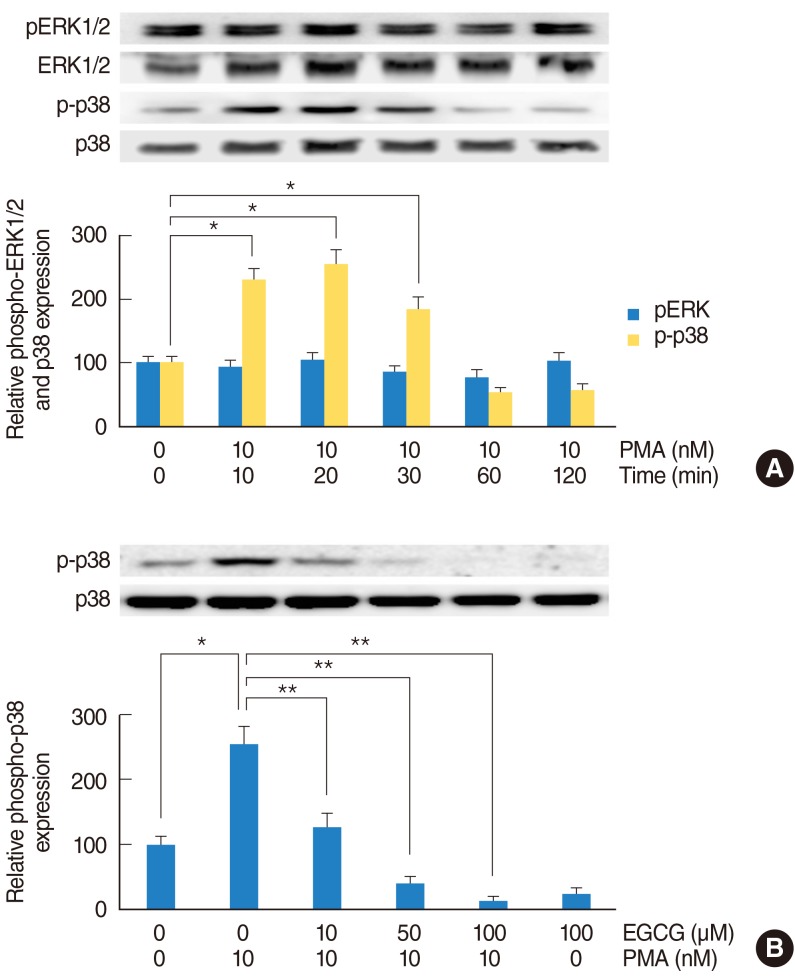
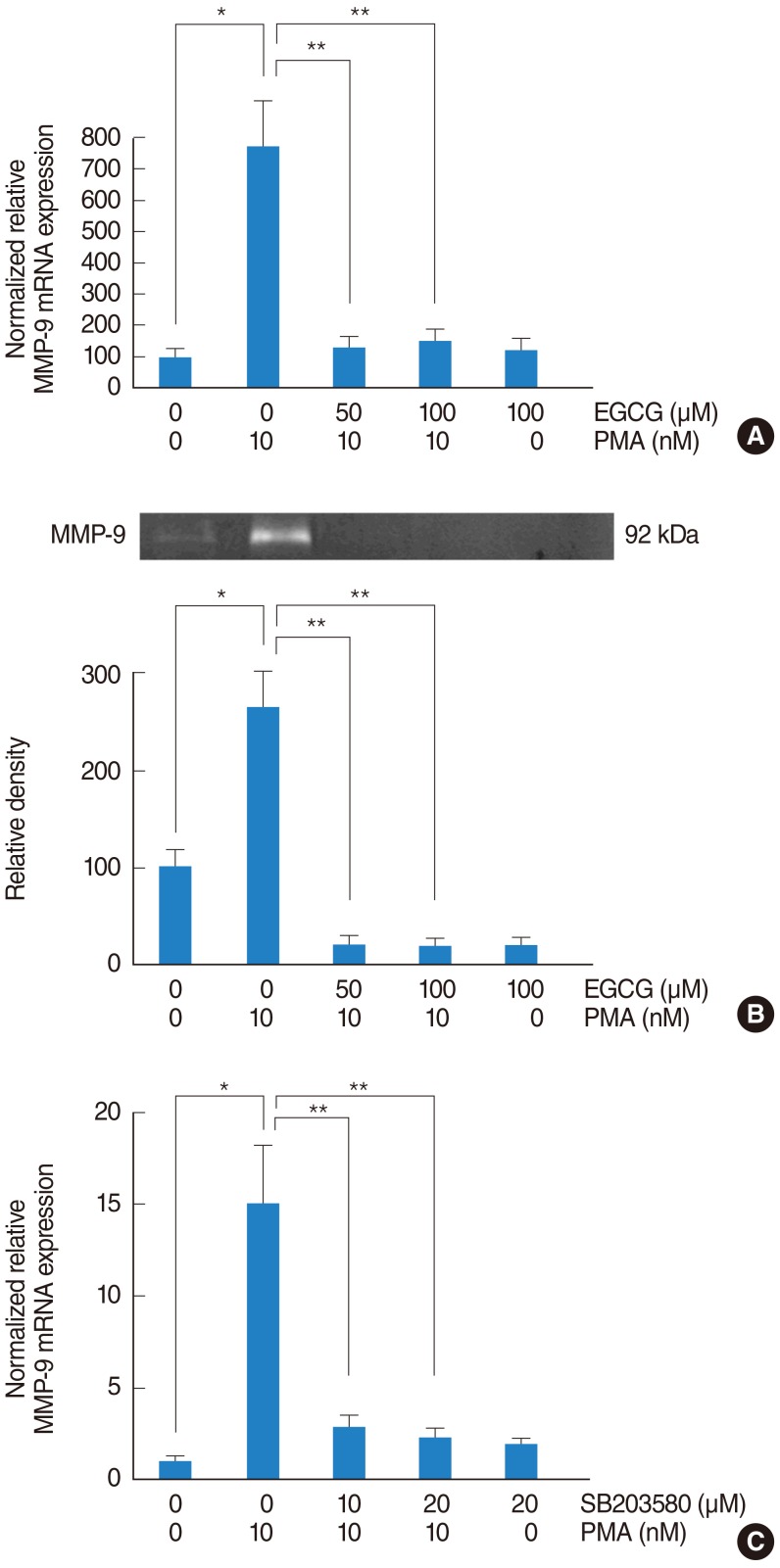
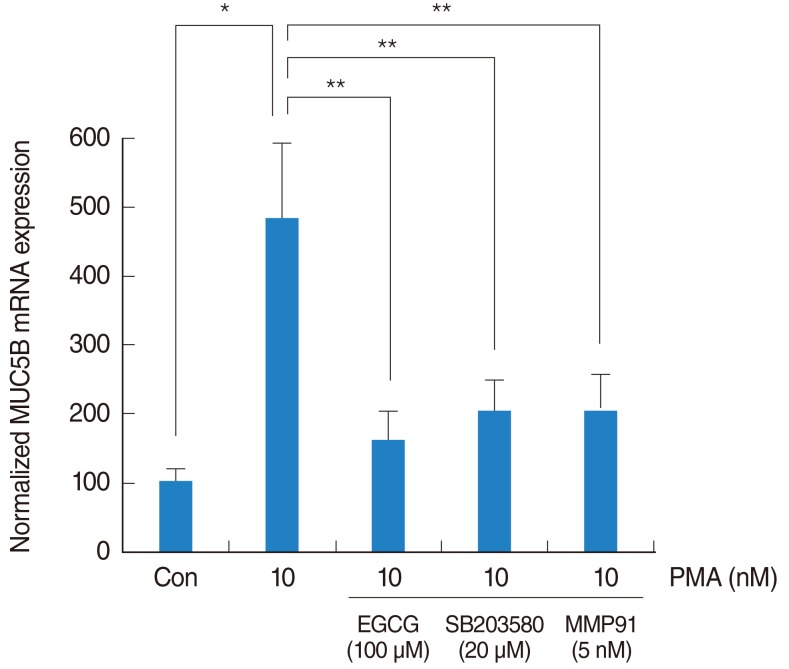
In NCI-H292 airway epithelial cells, PMA induced MUC5B expression, phosphorylation of p38 mitogen-activated protein kinase (MAPK), and matrix metalloproteinase-9 (MMP-9) expression and protein activity. EGCG significantly decreased PMA-induced MUC5B expression, phosphorylation of p38 MAPK, and MMP-9 expression and protein activity. SB203580 (p38 MAPK inhibitor) significantly decreased PMA-induced MMP-9 expression. In addition, SB203580 and MMP-9 I (MMP-9 inhibitor) significantly decreased PMA-induced MUC5B expression.
CONCLUSION
These results suggest that EGCG down-regulates PMA-induced MUC5B expression through the p38 MAPK dependent MMP-9 signaling pathway in human airway epithelial cells.
MeSH Terms
-
Catechin
Cholesterol
Epithelial Cells*
Gelatin
Humans*
Imidazoles
Immunoenzyme Techniques
Matrix Metalloproteinase 9
p38 Mitogen-Activated Protein Kinases
Phorbols
Phosphorylation
Protein Kinases
Pyridines
Real-Time Polymerase Chain Reaction
Tea
Catechin
Cholesterol
Gelatin
Imidazoles
Matrix Metalloproteinase 9
Phorbols
Protein Kinases
Pyridines
Tea
p38 Mitogen-Activated Protein Kinases
Figure
Reference
-
1. Williams OW, Sharafkhaneh A, Kim V, Dickey BF, Evans CM. Airway mucus: from production to secretion. Am J Respir Cell Mol Biol. 2006; 5. 34(5):527–536. PMID: 16415249.2. Ali MS, Pearson JP. Upper airway mucin gene expression: a review. Laryngoscope. 2007; 5. 117(5):932–938. PMID: 17473699.
Article3. Evans CM, Kim K, Tuvim MJ, Dickey BF. Mucus hypersecretion in asthma: causes and effects. Curr Opin Pulm Med. 2009; 1. 15(1):4–11. PMID: 19077699.
Article4. Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006; 1. 86(1):245–278. PMID: 16371599.
Article5. Yuan-Chen Wu D, Wu R, Reddy SP, Lee YC, Chang MM. Distinctive epidermal growth factor receptor/extracellular regulated kinase-independent and -dependent signaling pathways in the induction of airway mucin 5B and mucin 5AC expression by phorbol 12-myristate 13-acetate. Am J Pathol. 2007; 1. 170(1):20–32. PMID: 17200179.
Article6. Bae CH, Chen SM, Lee HM, Song SY, Kim YD. The effect of doxycycline on PMA-induced MUC5B expression via MMP-9 and p38 in NCI-H292 cells. Clin Exp Otorhinolaryngol. 2011; 12. 4(4):177–183. PMID: 22232712.
Article7. Raederstorff DG, Schlachter MF, Elste V, Weber P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J Nutr Biochem. 2003; 6. 14(6):326–332. PMID: 12873714.
Article8. Oliva J, Bardag-Gorce F, Tillman B, French SW. Protective effect of quercetin, EGCG, catechin and betaine against oxidative stress induced by ethanol in vitro. Exp Mol Pathol. 2011; 6. 90(3):295–299. PMID: 21352821.
Article9. Lee H, Bae JH, Lee SR. Protective effect of green tea polyphenol EGCG against neuronal damage and brain edema after unilateral cerebral ischemia in gerbils. J Neurosci Res. 2004; 9. 77(6):892–900. PMID: 15334607.
Article10. Bani D, Giannini L, Ciampa A, Masini E, Suzuki Y, Menegazzi M, et al. Epigallocatechin-3-gallate reduces allergen-induced asthma-like reaction in sensitized guinea pigs. J Pharmacol Exp Ther. 2006; 6. 317(3):1002–1011. PMID: 16525038.
Article11. Kim SH, Park HJ, Lee CM, Choi IW, Moon DO, Roh HJ, et al. Epigallocatechin-3-gallate protects toluene diisocyanate-induced airway inflammation in a murine model of asthma. FEBS Lett. 2006; 3. 580(7):1883–1890. PMID: 16516891.
Article12. Wang Y, Shen Y, Li K, Zhang P, Wang G, Gao L, et al. Role of matrix metalloproteinase-9 in lipopolysaccharide-induced mucin production in human airway epithelial cells. Arch Biochem Biophys. 2009; 6. 486(2):111–118. PMID: 19389382.
Article13. Hewson CA, Edbrooke MR, Johnston SL. PMA induces the MUC5AC respiratory mucin in human bronchial epithelial cells, via PKC, EGF/TGF-alpha, Ras/Raf, MEK, ERK and Sp1-dependent mechanisms. J Mol Biol. 2004; 11. 344(3):683–695. PMID: 15533438.14. Wu DY, Wu R, Chen Y, Tarasova N, Chang MM. PMA stimulates MUC5B gene expression through an Sp1-based mechanism in airway epithelial cells. Am J Respir Cell Mol Biol. 2007; 11. 37(5):589–597. PMID: 17600309.15. Ko FW, Diba C, Roth M, McKay K, Johnson PR, Salome C, et al. A comparison of airway and serum matrix metalloproteinase-9 activity among normal subjects, asthmatic patients, and patients with asthmatic mucus hypersecretion. Chest. 2005; 6. 127(6):1919–1927. PMID: 15947303.
Article16. Deshmukh HS, Shaver C, Case LM, Dietsch M, Wesselkamper SC, Hardie WD, et al. Acrolein-activated matrix metalloproteinase 9 contributes to persistent mucin production. Am J Respir Cell Mol Biol. 2008; 4. 38(4):446–454. PMID: 18006877.
Article17. Ren S, Guo LL, Yang J, Liu DS, Wang T, Chen L, et al. Doxycycline attenuates acrolein-induced mucin production, in part by inhibiting MMP-9. Eur J Pharmacol. 2011; 1. 650(1):418–423. PMID: 21036164.
Article18. Qin S, Alcorn JF, Craigo JK, Tjoeng C, Tarwater PM, Kolls JK, et al. Epigallocatechin-3-gallate reduces airway inflammation in mice through binding to proinflammatory xhemokines and inhibiting inflammatory cell recruitment. J Immunol. 2011; 3. 186(6):3693–3700. PMID: 21307292.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Doxycycline on PMA-Induced MUC5B Expression via MMP-9 and p38 in NCI-H292 Cells
- Roflumilast Attenuates MUC5AC and MUC5B Expression in Airway Epithelial Cells
- Effect of Betulinic Acid on MUC5AC and MUC5B Expression in Airway Epithelial Cells
- Effect of Multi-Walled Carbon Nanotubes on MUC5AC and MUC5B Expression in Airway Epithelial Cells
- Roxithromycin Suppresses MUC5B/8 Mucin Genes and Mucin Production in Airway Epithelial Cells