Clin Exp Otorhinolaryngol.
2011 Dec;4(4):177-183.
The Effect of Doxycycline on PMA-Induced MUC5B Expression via MMP-9 and p38 in NCI-H292 Cells
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Yeungnam University College of Medicine, Daegu, Korea. ydkim@med.yu.ac.kr
- 2Department of Otorhinolaryngology-Head and Neck Surgery, Korea University College of Medicine, Seoul, Korea.
- 3Center for Respiratory Disease, Yeungnam University Medical Center, Daegu, Korea.
Abstract
OBJECTIVES
Doxycycline is commonly used in medicine for its bacteriostatic antimicrobial properties. Recent studies have reported that doxycycline also has anti-inflammatory effects. Matrix metalloproteinase (MMP)-9 has been found to be involved in the physiological and pathological process of inflammatory airway disease. Phorbol 12-myristate 13-acetate (PMA), a protein kinase C activator, is known to stimulate the expression of MMP and mucin genes in the airway and intestinal epithelial cells. Therefore, the effects and signal pathways of doxycycline on PMA-induced MUC5B expression dependent MMP-9 in human airway epithelial cells were investigated.
METHODS
In human NCI-H292 airway epithelial cells, MUC5B and MMP-9 mRNA expression, MUC5B protein expression, and MMP-9 protein activity after the treatment with PMA, MMP-9 or doxycycline were determined by reverse transcriptase-polymerase chain reaction, enzyme immunoassay, gelatin zymography, and Western blot analysis.
RESULTS
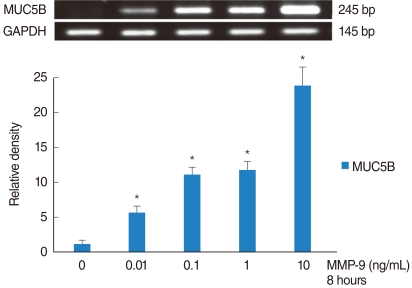
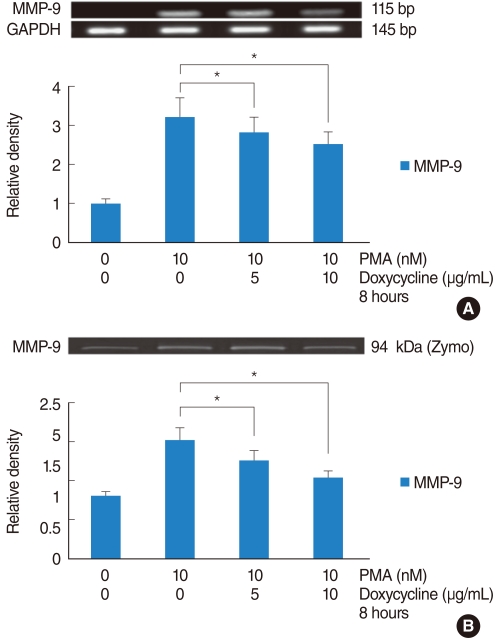
PMA increased MMP-9 and MUC5B expression. MMP-9 increased MUC5B expression. Doxycycline inhibited PMA-induced MUC5B expression, and PMA-induced MMP-9 mRNA expression and protein activity. Doxycycline inhibited phosphorylation of p38 induced by PMA and MMP-9.
CONCLUSION
The results of this study suggest that doxycycline inhibited PMA-induced MUC5B mRNA expression and protein production through the MMP-9 and p38 pathways in human NCI-H292 airway epithelial cells.
Keyword
MeSH Terms
-
Blotting, Western
Doxycycline
Epithelial Cells
Gelatin
Humans
Immunoenzyme Techniques
Inflammation
Matrix Metalloproteinase 9
Mucins
Phorbols
Phosphorylation
Protein Kinase C
RNA, Messenger
Signal Transduction
Tetradecanoylphorbol Acetate
Thiram
Doxycycline
Gelatin
Matrix Metalloproteinase 9
Mucins
Phorbols
Protein Kinase C
RNA, Messenger
Tetradecanoylphorbol Acetate
Thiram
Figure
Reference
-
1. Ali MS, Pearson JP. Upper airway mucin gene expression: a review. Laryngoscope. 2007; 5. 117(5):932–938. PMID: 17473699.
Article2. Caramori G, Casolari P, Di Gregorio C, Saetta M, Baraldo S, Boschetto P, et al. MUC5AC expression is increased in bronchial submucosal glands of stable COPD patients. Histopathology. 2009; 9. 55(3):321–331. PMID: 19723147.
Article3. Turner J, Jones CE. Regulation of mucin expression in respiratory diseases. Biochem Soc Trans. 2009; 8. 37(Pt 4):877–881. PMID: 19614611.
Article4. Ohbayashi H, Shimokata K. Matrix metalloproteinase-9 and airway remodeling in asthma. Curr Drug Targets Inflamm Allergy. 2005; 4. 4(2):177–181. PMID: 15853739.5. Sampsonas F, Kaparianos A, Lykouras D, Karkoulias K, Spiropoulos K. DNA sequence variations of metalloproteinases: their role in asthma and COPD. Postgrad Med J. 2007; 4. 83(978):244–250. PMID: 17403951.
Article6. Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol. 2003; 1. 28(1):12–24. PMID: 12495928.7. Wu DY, Wu R, Chen Y, Tarasova N, Chang MM. PMA stimulates MUC5B gene expression through an Sp1-based mechanism in airway epithelial cells. Am J Respir Cell Mol Biol. 2007; 11. 37(5):589–597. PMID: 17600309.8. Roomi MW, Monterrey JC, Kalinovsky T, Rath M, Niedzwiecki A. Patterns of MMP-2 and MMP-9 expression in human cancer cell lines. Oncol Rep. 2009; 5. 21(5):1323–1333. PMID: 19360311.
Article9. Lee HW, Ahn DH, Crawley SC, Li JD, Gum JR Jr, Basbaum CB, et al. Phorbol 12-myristate 13-acetate up-regulates the transcription of MUC2 intestinal mucin via Ras, ERK, and NF-kappa B. J Biol Chem. 2002; 9. 277(36):32624–32631. PMID: 12077118.10. Yuan-Chen Wu D, Wu R, Reddy SP, Lee YC, Chang MM. Distinctive epidermal growth factor receptor/extracellular regulated kinase-independent and -dependent signaling pathways in the induction of airway mucin 5B and mucin 5AC expression by phorbol 12-myristate 13-acetate. Am J Pathol. 2007; 1. 170(1):20–32. PMID: 17200179.
Article11. Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006; 2. 54(2):258–265. PMID: 16443056.
Article12. Kim HS, Luo L, Pflugfelder SC, Li DQ. Doxycyline inhibits TGF-beta1-induced MMP-9 via Smad and MAPK pathway in human corneal epithelial cell. Invest Ophthalmol Vis Sci. 2005; 3. 46(3):840–848. PMID: 15728539.13. Desseyn JL, Guyonnet-Duperat V, Porchet N, Aubert JP, Laine A. Human mucin gene MUC5B, the 10.7-kb large central exon encodes various alternate subdomains resulting in a super-repeat: structural evidence for a 11p15.5 gene family. J Biol Chem. 1997; 2. 272(6):3168–3178. PMID: 9013550.14. Kamio K, Matsushita I, Hijikata M, Kobashi Y, Tanaka G, Nakata K, et al. Promoter analysis and aberrant expression of the MUC5B gene in diffuse panbronchiolitis. Am J Respir Crit Care Med. 2005; 5. 171(9):949–957. PMID: 15709052.15. Henke MO, John G, Germann M, Lindemann H, Rubin BK. MUC5AC and MUC5B mucins increase in cystic fibrosis airway secretions during pulmonary exacerbation. Am J Respir Crit Care Med. 2007; 4. 175(8):816–821. PMID: 17255563.
Article16. Bernacki SH, Nelson AL, Abdullah L, Sheehan JK, Harris A, Davis CW, et al. Mucin gene expression during differentiation of human airway epithelia in vitro: muc4 and muc5b are strongly induced. Am J Respir Cell Mol Biol. 1999; 4. 20(4):595–604. PMID: 10100990.17. Garg P, Ravi A, Patel NR, Roman J, Gewirtz AT, Merlin D, et al. Matrix metalloproteinase-9 regulates MUC-2 expression through its effect on goblet cell differentiation. Gastroenterology. 2007; 5. 132(5):1877–1889. PMID: 17484881.
Article18. Deshmukh HS, Shaver C, Case LM, Dietsch M, Wesselkamper SC, Hardie WD, et al. Acrolein-activated matrix metalloproteinase 9 contributes to persistent mucin production. Am J Respir Cell Mol Biol. 2008; 4. 38(4):446–454. PMID: 18006877.
Article19. Hewson CA, Edbrooke MR, Johnston SL. PMA induces the MUC5AC respiratory mucin in human bronchial epithelial cells, via PKC, EGF/TGF-alpha, Ras/Raf, MEK, REK and Sp1-dependent mechanisms. J Mol Biol. 2004; 11. 344(3):683–695. PMID: 15533438.20. Wang Y, Shen Y, Li K, Zhang P, Wang G, Gao L, et al. Role of matrix metalloproteinase-9 in lipopolysaccharide-induced mucin production in human airway epithelial cells. Arch Biochem Biophys. 2009; 6. 486(2):111–118. PMID: 19389382.
Article21. Ren S, Guo LL, Yang J, Liu DS, Wang T, Chen L, et al. Doxycycline attenuates acrolein-induced mucin production, in part by inhibiting MMP-9. Eur J Pharmacol. 2011; 1. 650(1):418–423. PMID: 21036164.
Article22. Moon UY, Kim CH, Choi JY, Kim YJ, Choi YH, Yoon HG, et al. AP2alpha is essential for MUC8 gene expression in human airway epithelial cells. J Cell Biochem. 2010; 8. 110(6):1386–1398. HYPERLINK "http://www.ncbi.nlm.nih.gov/pubmed/20564234". PMID: 20564234.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Epigallocatechin-3-Gallate on PMA-Induced MUC5B Expression in Human Airway Epithelial Cells
- Roflumilast Attenuates MUC5AC and MUC5B Expression in Airway Epithelial Cells
- Effect of Betulinic Acid on MUC5AC and MUC5B Expression in Airway Epithelial Cells
- Effect of Udenafil on MUC5B Expression in Human Airway Epithelial Cells
- Effects of Cynaroside, Cynarin and Linarin on Secretion, Production and Gene Expression of Airway MUC5AC Mucin in NCI-H292 Cells