J Korean Surg Soc.
2011 Mar;80(3):194-203. 10.4174/jkss.2011.80.3.194.
Hypoxia Activates Toll-like Receptor 4 Signaling in Primary Mouse Hepatocytes Through the Receptor Clustering within Lipid Rafts
- Affiliations
-
- 1Department of Surgery, Eulji General Hospital, Eulji University School of Medicine, Seoul, Korea. kdh2109@eulji.ac.kr
- 2Department of Surgery, University of Pittsburgh, Pittsburgh, PA 15213, US.
- KMID: 1963583
- DOI: http://doi.org/10.4174/jkss.2011.80.3.194
Abstract
- PURPOSE
Transient hypoxia is an initial event that accentuates ischemia/reperfusion (I/R) injury in the liver. Hepatic ischemia/reperfusion (I/R) injury is largely related to innate immunity via Toll-like receptor 4 (TLR4) signaling. However, the mechanism by which hypoxia could lead to activate TLR4 signaling remains unclear. Therefore, the aim of this experimental study investigates how TLR4 signalling is activated by hypoxia.
METHODS
Hepatocytes were isolated from male wild-type (C57BL/6) mice (8~12 weeks old) by an in situ collagenase (Type IV, Sigma-Aldrich) perfusion technique. In this study, using primary mouse hepatocytes in culture to 1% oxygen, detection of TLR4 translocation to the lipid rafts on the cell membrane by immunofluorescence staining and immunoblotting was saught.
RESULTS
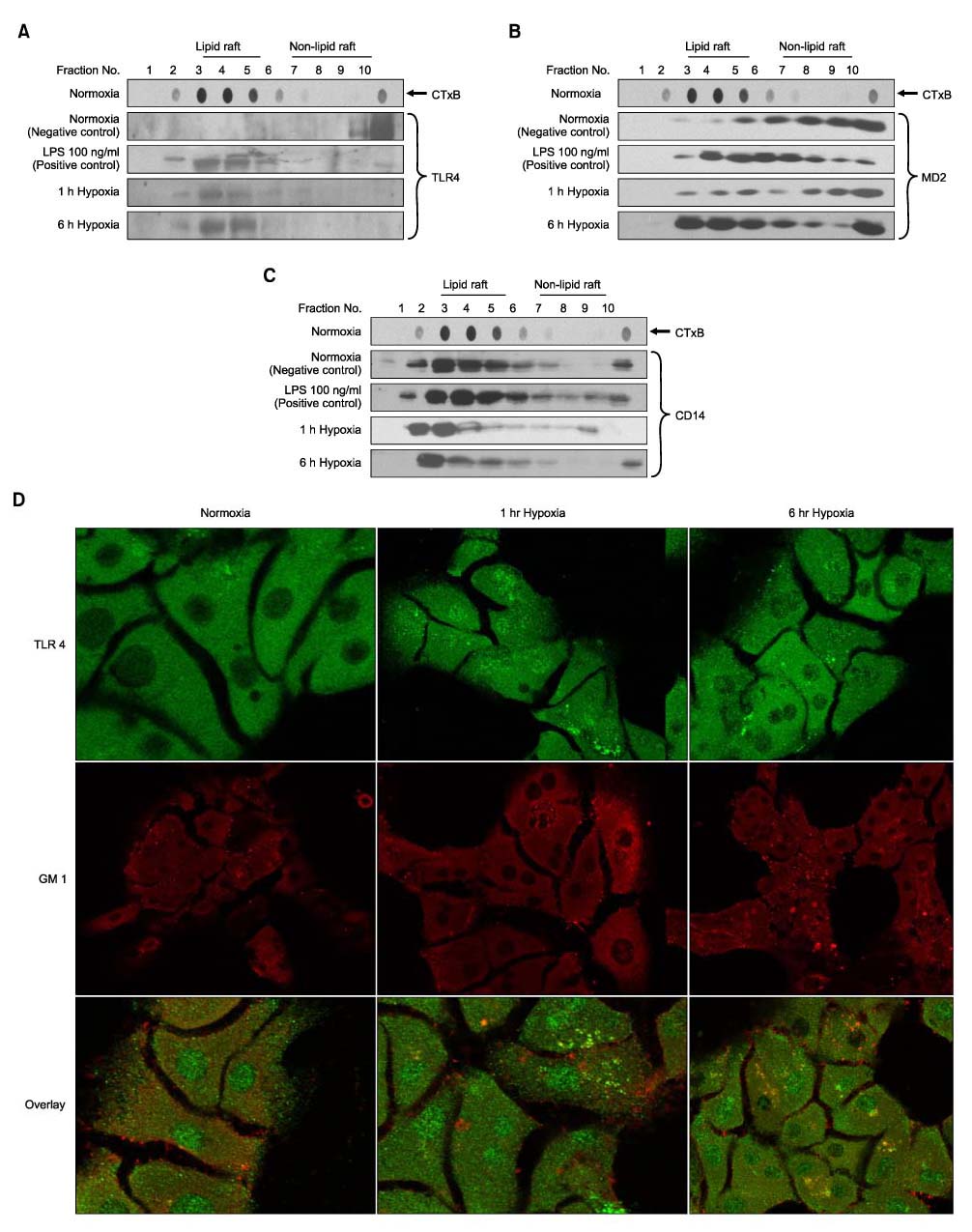
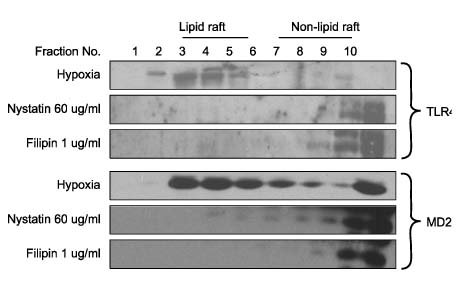
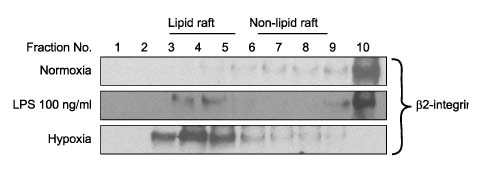
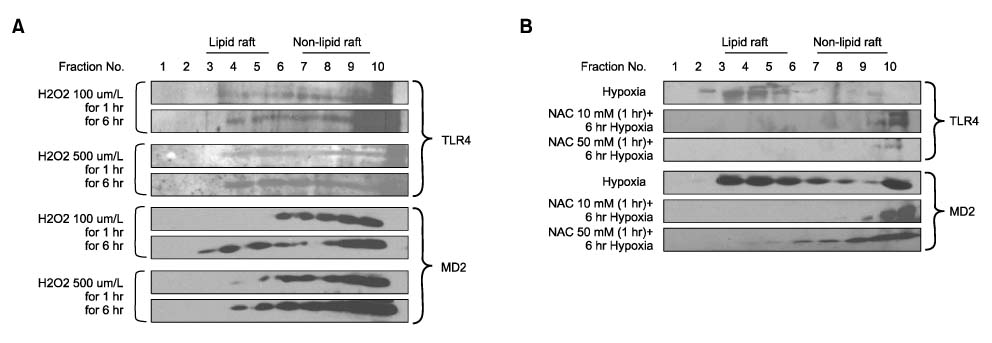
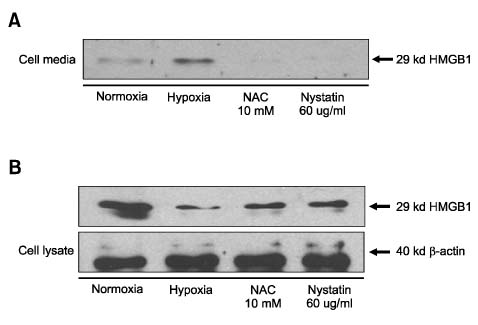
Hypoxia caused TLR4/MD2 and beta2-Integrin (CD11b/CD18) translocation to lipid rafts associated with CD14 in hepatocytes. The cholesterol sequestering agent, Nystatin and Filipin prevented hypoxia-induced TLR4/MD2 translocation to lipid rafts. Consistent with a role for oxidative stress in this effect, in vitro H2O2 treatment of hepatocytes similarly caused TLR4/MD2 translocation to lipid rafts. In addition, translocation of hypoxia-induced TLR4 complex was inhibited by N-acetylcysteine (NAC) demonstrating that the activation of TLR4 signaling is dependent on ROS. Further, the cholesterol sequestering agent, nystatin, prevented hypoxia-induced high mobility group box 1 (HMGB1) release in hepatocytes.
CONCLUSION
These results suggest that ROS dependent TLR4 signaling is achieved following receptor translocation to the lipid raft in hepatocytes. We hypothesized that this mechanism is required for the release of HMGB1, an early mediator of injury and inflammation in hepatic I/R injury.
Keyword
MeSH Terms
-
Acetylcysteine
Animals
Anoxia*
Cell Membrane
Cholesterol
Cluster Analysis*
Collagenases
Filipin
Fluorescent Antibody Technique
Hepatocytes*
HMGB1 Protein
Humans
Immunity, Innate
Immunoblotting
Inflammation
Liver
Male
Mice*
Nystatin
Oxidative Stress
Oxygen
Perfusion
Sequestering Agents
Toll-Like Receptor 4*
Acetylcysteine
Cholesterol
Collagenases
Filipin
HMGB1 Protein
Nystatin
Oxygen
Sequestering Agents
Toll-Like Receptor 4
Figure
Reference
-
1. Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury-a fresh look. Exp Mol Pathol. 2003. 74:86–93.2. Ock J, Cho H, Hong S, Kim IK, Suk K. Hypoxia as an initiator of neuroinflammation: microglial connections. Curr Neuropharmacol. 2005. 3:183–191.3. Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004. 4:499–511.4. Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007. 204:2913–2923.5. Arumugam TV, Okun E, Tang SC, Thundyil J, Taylor SM, Woodruff TM. Toll-like receptors in ischemia-reperfusion injury. Shock. 2009. 32:4–16.6. Helms JB, Zurzolo C. Lipids as targeting signals: lipid rafts and intracellular trafficking. Traffic. 2004. 5:247–254.7. Hao M, Mukherjee S, Maxfield FR. Cholesterol depletion induces large scale domain segregation in living cell membranes. Proc Natl Acad Sci USA. 2001. 98:13072–13077.8. Kabouridis PS. Lipid rafts in T cell receptor signalling. Mol Membr Biol. 2006. 23:49–57.9. Hornef MW, Normark BH, Vandewalle A, Normark S. Intracellular recognition of lipopolysaccharide by toll-like receptor 4 in intestinal epithelial cells. J Exp Med. 2003. 198:1225–1235.10. Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine tolllike receptor 2. J Immunol. 2000. 165:618–622.11. West MA, Billiar TR, Curran RD, Hyland BJ, Simmons RL. Evidence that rat Kupffer cells stimulate and inhibit hepatocyte protein synthesis in vitro by different mechanism. Gastroenterology. 1989. 96:1572–1582.12. Lai EC. Lipid rafts make for slippery platforms. J Cell Biol. 2003. 162:365–370.13. Triantafilou M, Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 2002. 23:301–304.14. Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci. 2002. 115:2603–2611.15. Marmor MD, Julius M. Role for lipid rafts in regulating interleukin-2 receptor signaling. Blood. 2001. 98:1489–1497.16. Miyake K. Endotoxin recognition molecules, Toll-like receptor 4-MD2. Semin Immunol. 2004. 16:11–16.17. Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992. 68:673–682.18. Todd RF III, Petty HR. Beta 2 (CD11/CD18) integrins can serve as signaling partners for other leukocyte receptors. J Lab Clin Med. 1997. 129:492–498.19. Perera PY, Mayadas TN, Takeuchi O, Akira S, Zaks-Zilberman M, Goyert SM, et al. CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J Immunol. 2001. 166:574–581.20. Jeyabalan G, Tsung A, Billiar TR. Linking proximal and downstream signaling events in hepatic ischemia/reperfusion injury. Biochem Soc Trans. 2006. 34:957–959.21. Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005. 201:1135–1143.22. Fan J, Li Y, Levy RM, Fan JJ, Hackam DJ, Vodovotz Y, et al. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J Immunol. 2007. 178:6573–6580.23. Miyake K. Roles for accessory molecules in microbial recognition by Toll-like receptors. J Endotoxin Res. 2006. 12:195–204.24. Szabo G, Dolganiuc A, Dai Q, Pruett SB. TLR4, ethanol, and lipid rafts: a new mechanism of ethanol action with implications for other receptor-mediated effects. J Immunol. 2007. 178:1243–1249.25. Dawson TL, Gores GL, Nieminen AL, Herman B, Lemasters JJ. Mitochondria as a source of reactive oxygen species during reductive stress in rat hepatocytes. Am J Physiol. 1993. 264:C961–C967.26. Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1 during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000. 275:25130–25138.27. Abdelrahman M, Mazzon E, Bauer M, Bauer J, Delbosc S, Cristol JP, et al. Inhibitors of NADPH oxidase reduce the organ injury in hemorrhagic shock. Shock. 2005. 23:107–114.28. Ulloa L, Messmer D. High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev. 2006. 17:189–201.29. Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med. 2004. 255:320–331.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nucleic Acid Recognition and Signaling by Toll-like Receptor 9: Compartment-dependent Regulation
- Lipopolysaccharide: Basic Biochemistry, Intracellular Signaling, and Physiological Impacts in the Gut
- Toll-like receptor 4 antagonist and obesity associated kidney disease: Where should we go from here?
- Clinical Significance of Toll-Like Receptor and Toll-Like Receptor Blocker
- Targeting Microglial and Neuronal Toll-like Receptor 2 in Synucleinopathies