Yonsei Med J.
2013 Sep;54(5):1186-1193. 10.3349/ymj.2013.54.5.1186.
Growth Inhibition of Hepatocellular Carcinoma Huh7 Cells by Lactobacillus casei Extract
- Affiliations
-
- 1Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea.
- 2Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 3Interdisciplinary Program of Integrated Biotechnology, Sogang University, Seoul, Korea. hyuncheol@sogang.ac.kr
- 4Department of Chemical and Biomolecular Engineering, Sogang University, Seoul, Korea.
- KMID: 1793166
- DOI: http://doi.org/10.3349/ymj.2013.54.5.1186
Abstract
- PURPOSE
Lactobacillus casei (L. casei) is known to exert anti-proliferation effects on many types of cancer cells. However, the effect of L. casei on liver cancer has not been reported. Accordingly, the aim of this study was to determine the anti-cancer effect of L. casei extract on Huh7 cells.
MATERIALS AND METHODS
L. casei ATCC393 extract was prepared and purified. After the treatment of L. casei extract on Huh7 cells, cell viability, cell cycle arrest and cell death were analyzed by flow cytometry. The expression levels of tumor necrosis factor-alpha receptor 1 (TNFR1) and death receptor 3 (DR3) mRNA related with extrinsic apoptosis were assessed by reverse transcription polymerase chain reaction. Additionally, P21 and P27 cell cycle proteins as well as Caspase-3, -8, -9, phospho-Bad and Bcl-2 apoptosis proteins were analyzed by western blot analysis. To determine the effect of L. casei extract on cancer stem-like cells, we analyzed changes in side population fraction through flow cytometry.
RESULTS
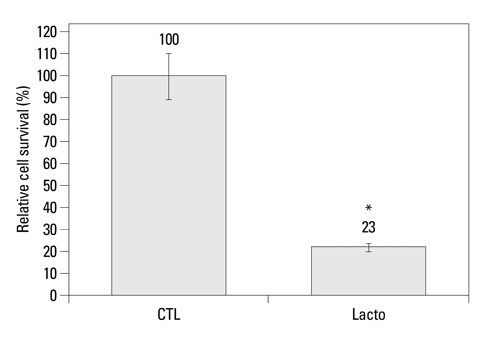
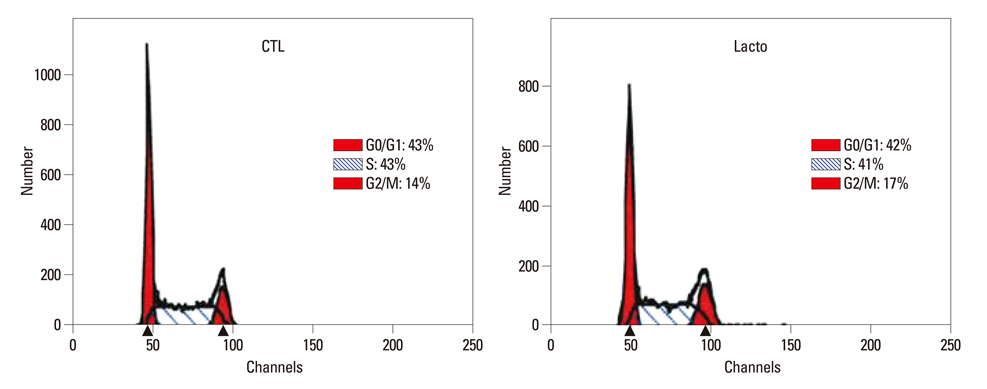
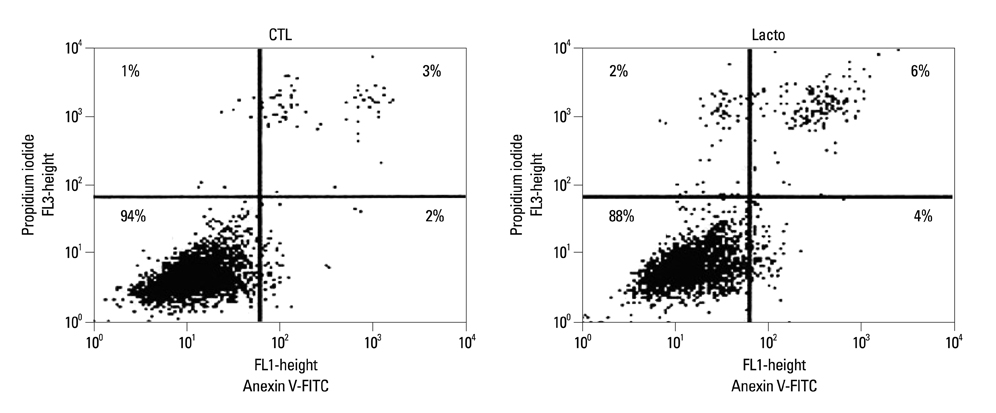
The cell viability of Huh7 cells treated with L. casei extract was decreased by 77%, potentially owing to increases in the rates of Huh7 cells arrested in the G2/M phase (3% increase) and that underwent apoptosis (6% increase). The expression levels of TNFR1 and DR3 mRNA, as well as P21 and P27 cell cycle proteins, were increased. Meanwhile, the expressions of caspase-8, -9, phospho-Bad and Bcl-2 proteins decreased. However, in the case of side population cells, no remarkable changes were observed.
CONCLUSION
L. casei extract exerts a potent anti-tumor effect on the viability of liver cancer cells, although not on cancer stem-like cells.
MeSH Terms
-
Apoptosis/drug effects
Carcinoma, Hepatocellular/*pathology
Caspase 8/metabolism
Caspase 9/metabolism
Cell Cycle Checkpoints/drug effects
Cell Extracts/*pharmacology
Cell Line, Tumor
Cell Proliferation/drug effects
Cyclin-Dependent Kinase Inhibitor p21/metabolism
Cyclin-Dependent Kinase Inhibitor p27/metabolism
Cytostatic Agents/*pharmacology
Flow Cytometry
Gene Expression Regulation, Neoplastic/drug effects
Humans
Lactobacillus casei/*chemistry
Liver Neoplasms/*pathology
Proto-Oncogene Proteins c-bcl-2/metabolism
RNA, Messenger/metabolism
Receptors, Tumor Necrosis Factor, Member 25/metabolism
Receptors, Tumor Necrosis Factor, Type I/metabolism
bcl-Associated Death Protein/metabolism
bcl-Associated Death Protein
Caspase 8
Caspase 9
Cell Extracts
Cyclin-Dependent Kinase Inhibitor p21
Cyclin-Dependent Kinase Inhibitor p27
Cytostatic Agents
Proto-Oncogene Proteins c-bcl-2
RNA, Messenger
Receptors, Tumor Necrosis Factor, Member 25
Receptors, Tumor Necrosis Factor, Type I
Figure
Reference
-
1. Kato I, Endo-Tanaka K, Yokokura T. Suppressive effects of the oral administration of Lactobacillus casei on type II collagen-induced arthritis in DBA/1 mice. Life Sci. 1998; 63:635–644.
Article2. Matsuzaki T, Nagata Y, Kado S, Uchida K, Kato I, Hashimoto S, et al. Prevention of onset in an insulin-dependent diabetes mellitus model, NOD mice, by oral feeding of Lactobacillus casei. APMIS. 1997; 105:643–649.
Article3. Matsuzaki T, Takagi A, Ikemura H, Matsuguchi T, Yokokura T. Intestinal microflora: probiotics and autoimmunity. J Nutr. 2007; 137:3 Suppl 2. 798S–802S.
Article4. Matsumoto S, Hara T, Nagaoka M, Mike A, Mitsuyama K, Sako T, et al. A component of polysaccharide peptidoglycan complex on Lactobacillus induced an improvement of murine model of inflammatory bowel disease and colitis-associated cancer. Immunology. 2009; 128:1 Suppl. e170–e180.5. Haro C, Zelaya H, Lazarte S, Alvarez S, Agüero G. Lactobacillus casei: influence on the innate immune response and haemostatic alterations in a liver-injury model. Can J Microbiol. 2009; 55:648–656.
Article6. Pawłowska J, Klewicka E, Czubkowski P, Motyl I, Jankowska I, Libudzisz Z, et al. Effect of Lactobacillus casei DN-114001 application on the activity of fecal enzymes in children after liver transplantation. Transplant Proc. 2007; 39:3219–3221.
Article7. Kato I, Kobayashi S, Yokokura T, Mutai M. Antitumor activity of Lactobacillus casei in mice. Gann. 1981; 72:517–523.8. Kato I, Endo K, Yokokura T. Effects of oral administration of Lactobacillus casei on antitumor responses induced by tumor resection in mice. Int J Immunopharmacol. 1994; 16:29–36.
Article9. Takagi A, Matsuzaki T, Sato M, Nomoto K, Morotomi M, Yokokura T. Inhibitory effect of oral administration of Lactobacillus casei on 3-methylcholanthrene-induced carcinogenesis in mice. Med Microbiol Immunol. 1999; 188:111–116.
Article10. Kato I, Yokokura T, Mutai M. Induction of tumoricidal peritoneal exudate cells by administration of Lactobacillus casei. Int J Immunopharmacol. 1985; 7:103–109.
Article11. Aso Y, Akaza H, Kotake T, Tsukamoto T, Imai K, Naito S. The BLP Study Group. Preventive effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double-blind trial. Eur Urol. 1995; 27:104–109.
Article12. Nanno M, Kato I, Kobayashi T, Shida K. Biological effects of probiotics: what impact does Lactobacillus casei shirota have on us? Int J Immunopathol Pharmacol. 2011; 24:1 Suppl. 45S–50S.13. Hathout AS, Mohamed SR, El-Nekeety AA, Hassan NS, Aly SE, Abdel-Wahhab MA. Ability of Lactobacillus casei and Lactobacillus reuteri to protect against oxidative stress in rats fed aflatoxins-contaminated diet. Toxicon. 2011; 58:179–186.
Article14. Wang DH, Koehler SM, Mariash CN. Detecting graves' disease: presentations in young athletes. Phys Sportsmed. 1996; 24:35–40.15. Zhang L, Zhang Y, Zhang L, Yang X, Lv Z. Lupeol, a dietary triterpene, inhibited growth, and induced apoptosis through down-regulation of DR3 in SMMC7721 cells. Cancer Invest. 2009; 27:163–170.
Article16. Merritt WM, Lin YG, Spannuth WA, Fletcher MS, Kamat AA, Han LY, et al. Effect of interleukin-8 gene silencing with liposome-encapsulated small interfering RNA on ovarian cancer cell growth. J Natl Cancer Inst. 2008; 100:359–372.
Article17. Chilampalli C, Guillermo R, Kaushik RS, Young A, Chandrasekher G, Fahmy H, et al. Honokiol, a chemopreventive agent against skin cancer, induces cell cycle arrest and apoptosis in human epidermoid A431 cells. Exp Biol Med (Maywood). 2011; 236:1351–1359.
Article18. Kim JB, Ko E, Han W, Shin I, Park SY, Noh DY. Berberine diminishes the side population and ABCG2 transporter expression in MCF-7 breast cancer cells. Planta Med. 2008; 74:1693–1700.
Article19. Choi SS, Kim Y, Han KS, You S, Oh S, Kim SH. Effects of Lactobacillus strains on cancer cell proliferation and oxidative stress in vitro. Lett Appl Microbiol. 2006; 42:452–458.
Article20. Fichera GA, Giese G. Non-immunologically-mediated cytotoxicity of Lactobacillus casei and its derivative peptidoglycan against tumor cell lines. Cancer Lett. 1994; 85:93–103.
Article21. Kim JY, Woo HJ, Kim YS, Kim KH, Lee HJ. Cell cycle dysregulation induced by cytoplasm of Lactococcus lactis ssp lactis in SNUC2A, a colon cancer cell line. Nutr Cancer. 2003; 46:197–201.
Article22. Hosonuma S, Kobayashi Y, Kojo S, Wada H, Seino K, Kiguchi K, et al. Clinical significance of side population in ovarian cancer cells. Hum Cell. 2011; 24:9–12.
Article23. Moserle L, Ghisi M, Amadori A, Indraccolo S. Side population and cancer stem cells: therapeutic implications. Cancer Lett. 2010; 288:1–9.
Article24. Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, et al. A distinct "side population" of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A. 2004; 101:14228–14233.
Article25. Roy S, Kaur M, Agarwal C, Tecklenburg M, Sclafani RA, Agarwal R. p21 and p27 induction by silibinin is essential for its cell cycle arrest effect in prostate carcinoma cells. Mol Cancer Ther. 2007; 6:2696–2707.
Article26. Kim JY, Song EH, Lee HJ, Oh YK, Choi KH, Yu DY, et al. HBx-induced hepatic steatosis and apoptosis are regulated by TNFR1- and NF-kappaB-dependent pathways. J Mol Biol. 2010; 397:917–931.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of Aflatoxin Production of Aspergillus flavus by Lactobacillus casei
- Lactobacillus casei subspecies casei endocarditis: a case report
- Adhesion Activity of Lactobacillus plantarum PM 008 Isolated from Kimchi on the Intestine of Mice
- Effects of Lactobacillus casei and Aggregatibactor actinomycetemcomitans against Streptococcus mutans according to the Concentration of Sucrose
- Beneficial Effects of Lactobacillus casei ATCC 334 on Halitosis Induced by Periodontopathogens