J Korean Med Sci.
2012 Sep;27(9):987-992. 10.3346/jkms.2012.27.9.987.
Prostaglandin E2 and Interleukin-1beta Reduce E-cadherin Expression by Enhancing Snail Expression in Gastric Cancer Cells
- Affiliations
-
- 1Department of Surgery, Dankook University Hospital, Cheonan, Korea.
- 2Department of Pathology, Dongguk University College of Medicine, Gyeongju, Korea. taejung@mail.dongguk.ac.kr
- 3Department of Surgery, Dongguk University College of Medicine, Gyeongju, Korea.
- KMID: 1782129
- DOI: http://doi.org/10.3346/jkms.2012.27.9.987
Abstract
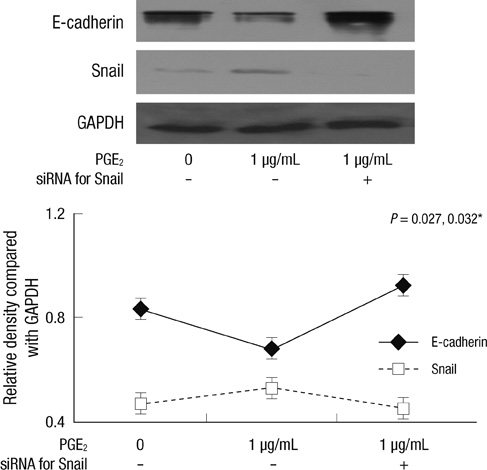
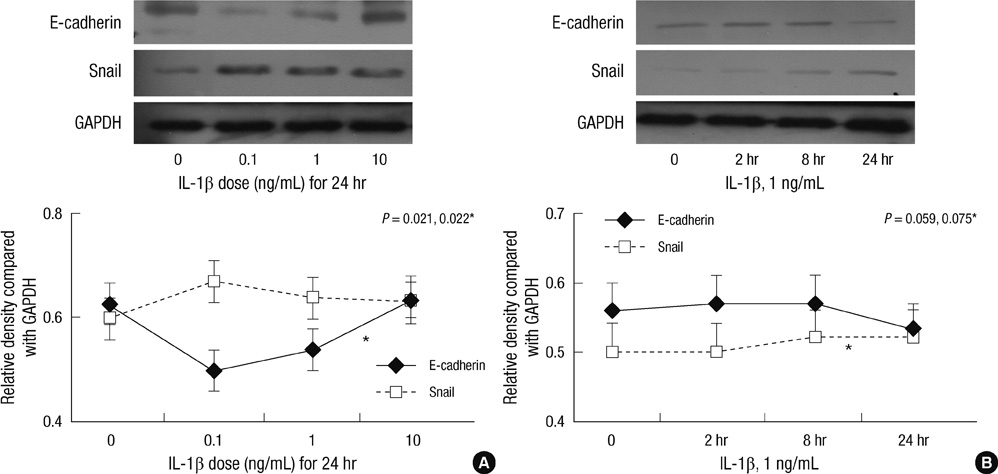
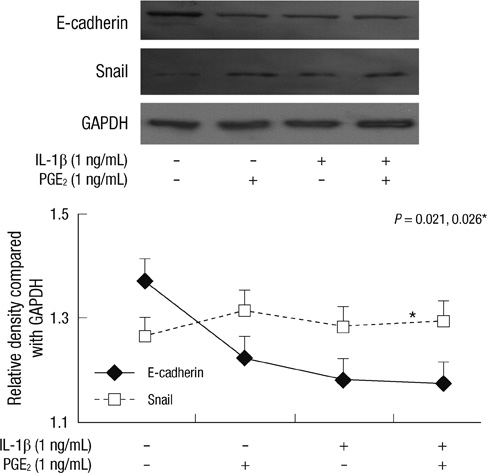
- Inflammation is closely related to the progression of cancer as well as tumorigenesis. Here, we investigated the effect of prostaglandin E2 (PGE2) and interleukin-1beta (IL-1beta) on E-cadherin expression in SNU719 gastric cancer cells. E-cadherin expression decreased as the dose or exposure time of PGE2 and IL-1beta increased, whereas Snail expression increased with dose or time of PGE2 and IL-1beta. E-cadherin expression reduced by PGE2 treatment increased after the transfection of Snail siRNA. Neutralization of IL-1beta using anti-IL-1beta antibody blocked the expression pattern of E-cadherin and Snail occurred by IL-1beta treatment. However, there was no synergic effect of IL-1beta and PGE2 on the expression pattern of E-cadherin and Snail. In conclusion, inflammatory mediators reduced E-cadherin expression by enhancing Snail expression in gastric cancer cells. Inflammation-induced transcriptional regulation of E-cadherin in gastric cancer has implications for targeted chemoprevention and therapy.
Keyword
MeSH Terms
-
Antibodies/immunology
Antineoplastic Agents/pharmacology
Cadherins/*metabolism
Cell Line, Tumor
Dinoprostone/*pharmacology
Gene Expression Regulation/*drug effects
Humans
Interleukin-1beta/immunology/*pharmacology
RNA Interference
RNA, Small Interfering/metabolism
Stomach Neoplasms/metabolism/pathology
Transcription Factors/antagonists & inhibitors/genetics/*metabolism
Antibodies
Antineoplastic Agents
Cadherins
Interleukin-1beta
RNA, Small Interfering
Transcription Factors
Dinoprostone
Figure
Reference
-
1. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008. 454:436–444.2. Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007. 39:305–318.3. Vleminckx K, Kemler R. Cadherins and tissue formation: integrating adhesion and signaling. Bioessays. 1999. 21:211–220.4. Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002. 2:442–454.5. Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancer. Am J Pathol. 1998. 153:333–339.6. Uefuji K, Ichikura T, Mochizuki H. Cyclooxygenase-2 expression is related to prostaglandin biosynthesis and angiogenesis in human gastric cancer. Clin Cancer Res. 2000. 6:135–138.7. Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995. 83:493–501.8. Tsujii M, Kawano S, DuBois RN. Cyclooxyenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci U S A. 1997. 94:3336–3340.9. Sitarz R, Leguit RJ, de Leng WW, Morsink FH, Polkowski WP, Maciejewski R, Offerhaus GJ, Milne AN. Cyclooxygenase-2 mediated regulation of E-cadherin occurs in conventional but not early-onset gastric cancer cell lines. Cell Oncol. 2009. 31:475–485.10. Dohadwala M, Yang SC, Luo J, Sharma S, Batra RK, Huang M, Lin Y, Goodglick L, Krysan K, Fishbein MC, et al. Cyclooxygenase-2-dependent regulation of E-cadherin: prostaglandin E2 induces transcriptional repressors ZEB1 and snail in non-small cell lung cancer. Cancer Res. 2006. 66:5338–5345.11. Jang TJ, Jeon KH, Jung KH. Cyclooxgenase-2 expression is related to the epithelial-to-mesenchymal transition in human colon cancers. Yonsei Med J. 2009. 50:818–824.12. Jang TJ, Cha WH, Lee KS. Reciprocal correlation between the expression of cyclooxygenase-2 and E-cadherin in human transitional cell carcinomas. Virchows Arch. 2010. 457:319–328.13. Yamaoka Y, Kita M, Kodama T, Sawai N, Kashima K, Imanishi J. Induction of various cytokines and development of severe mucosal inflammation by cagA gene positive Helicobacter pylori strains. Gut. 1997. 41:442–451.14. El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000. 404:398–402.15. Qian X, Huang C, Cho CH, Hui WM, Rashid A, Chan AO, et al. E-cadherin promoter hypermethylation induced by interleukin-1b treatment or H. pylori infection in human gastric cancer cell lines. Cancer Lett. 2008. 263:107–113.16. St. John MA, Dohadwala M, Luo J, Wang G, Lee G, Shih H, Heinrich E, Krysan K, Walser T, Hazra S, et al. Proinflammatory mediators upregulates Snail in head and neck squamous cell carcinoma. Clin Cancer Res. 2009. 15:6018–6027.17. Lopez-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009. 1:303–314.18. Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, Song X, Dvozkin T, Krelin Y, Voronov E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006. 25:387–408.19. Krelin Y, Voronov E, Dotan S, Elkabets M, Reich E, Fogel M, Huszar M, Iwakura Y, Segal S, Dinarello CA, et al. Interleukin-1beta-driven inflammation promotes the development and invasiveness of chemical carcinogen-induced tumors. Cancer Res. 2007. 67:1062–1071.20. McNamara D, El-Omar EM. Helicobacter pylori infection and the pathogenesis of gastric cancer: a paradigm for host-bacterial interactions. Dig Liver Dis. 2008. 40:504–509.21. Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008. 14:408–419.22. Song SH, Jong HS, Choi HH, Inoue H, Tanabe K, Kim NK, Bang YJ. Transcriptional silencing of cyclooxygenase-2 by hyper-methylation of the 5' CpG island in human gastric carcinoma cells. Cancer Res. 2001. 61:4628–4635.23. Mann JR, Backlund MG, Buchanan FG, Holla VR, Shi Q, Daikoku T, Dey SK, DuBois RN. Repression of prostaglandin dehydrogenase by epidermal growth factor and snail increases prostaglandin E2 and promotes cancer progression. Cancer Res. 2006. 66:6649–6656.24. Brouxhon S, Kyrkanides S, O'Banion MK, Johnson R, Pearce DA, Centola GM, Miller JN, McGrath KH, Erdle B, Scott G, et al. Sequential downregulation of E-cadherin with squamous cell carcinoma progression: Loss of E-cadherin via a prostaglandin E2-EP2-dependent posttranslational mechanism. Cancer Res. 2007. 67:7654–7664.25. Noda M, Tatsumi Y, Tomizawa M, Takama T, Mitsufuji S, Sugihara H, Kashima K, Hattori T. Effects of etodolac, a selective cyclooxygenase-2 inhibitor, on the expression of E-cadherin-catenin complexes in gastrointestinal cell lines. J Gastroenterol. 2002. 37:896–904.26. Ran JT, Zhou YN, Tang CW, Li HH, Li XW, Lu H. Short-term preoperative treatment of celecoxib, a selective cyclooxygenase-2 inhibitor, on E-cadherin expression in gastric carcinoma tissues. Ai Zheng. 2009. 28:361–365.27. Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Höfler H. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res. 1994. 54:3845–3852.28. Chen HC, Chu RY, Hsu PN, Hsu PI, Lu JY, Lai KH, Tseng HH, Chou NH, Huang MS, Tseng CJ, et al. Loss of E-cadherin expression correlates with poor differentiation and invasion into adjacent organs in gastric adenocarcinomas. Cancer Lett. 2003. 201:97–106.29. Kim TY, Jong HS, Jung Y, Kim TY, Kang GH, Bang YJ. DNA hypermethylation in gastric cancer. Aliment Pharmacol Ther. 2004. 20:S131–S142.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cyclooxygenase-2 Expression Is Related to the Epithelial-to-Mesenchymal Transition in Human Colon Cancers
- IL-1beta Mediated COX-2 Expression in Human Airway Epithelial Cells
- Trefoil Factor 1 Suppresses Epithelial-mesenchymal Transition through Inhibition of TGF-beta Signaling in Gastric Cancer Cells
- Interleukin-1beta-Mediated MUC5AC Gene Expression and Mucin Secretion via PKC-ERK/p38-COX-2-PGE2 in Human Airway Epithelial Cells
- Clinicopathologic significance of the expression of Snail in hepatocellular carcinoma