Chonnam Med J.
2020 Jan;56(1):20-26. 10.4068/cmj.2020.56.1.20.
Fluoxetine Induces Apoptotic and Oxidative Neuronal Death Associated with The Influx of Copper Ions in Cultured Neuronal Cells
- Affiliations
-
- 1Department of Pharmacology, Chonnam National University Medical School, Hwasun, Korea. jkkim57@jnu.ac.kr
- KMID: 2468142
- DOI: http://doi.org/10.4068/cmj.2020.56.1.20
Abstract
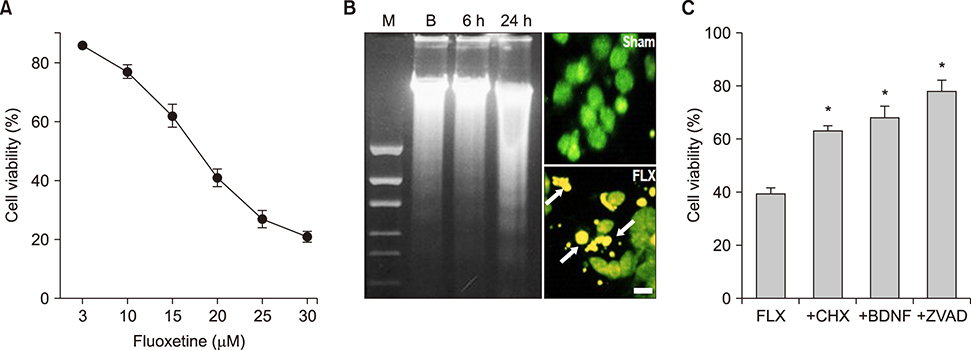
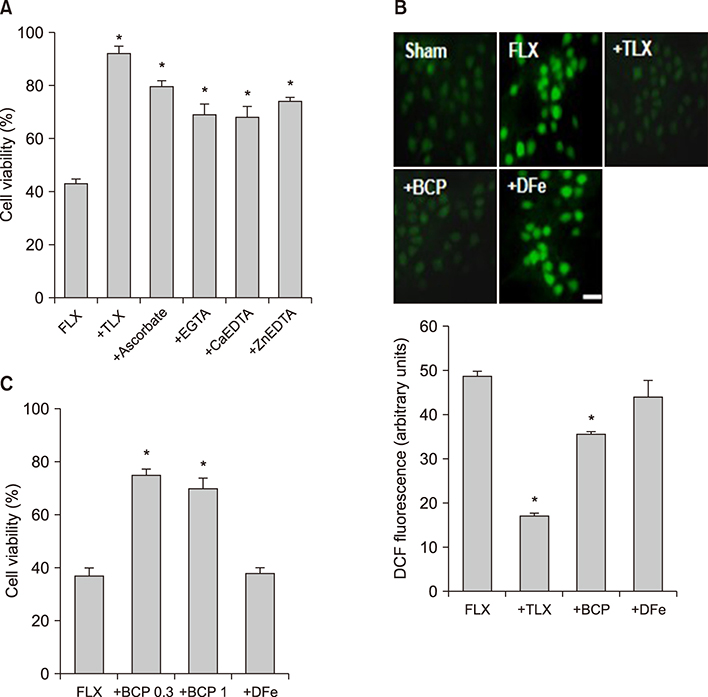
- We examined the effect of fluoxetine, a selective serotonin reuptake inhibitor antidepressant, on neuronal viability in mouse cortical near-pure neuronal cultures. Addition of fluoxetine to the media for 24 hours induced neuronal death in a concentration-dependent manner. To delineate the mechanisms of fluoxetine-induced neuronal death, we investigated the effects of trolox, cycloheximide (CHX), BDNF, z-VAD-FMK, and various metal-chelators on fluoxetine-induced neuronal death. Neuronal death was assessed by MTT assay. The addition of 20 µM fluoxetine to the media for 24 hours induced 60-70% neuronal death, which was associated with the hallmarks of apoptosis, chromatin condensation and DNA laddering. Fluoxetine-induced death was significantly attenuated by CHX, BDNF, or z-VAD-FMK. Treatment with antioxidants, trolox and ascorbate, also markedly attenuated fluoxetine-induced death. Interestingly, some divalent cation chelators (EGTA, Ca-EDTA, and Zn-EDTA) also markedly attenuated the neurotoxicity. Fluoxetine-induced reactive oxygen species (ROS) generation was measured using the fluorescent dye 2"²,7"²-dichlorofluorescin diacetate. Trolox and bathocuproine disulfonic acid (BCPS), a cell membrane impermeable copper ion chelator, markedly attenuated the ROS production and neuronal death. However, deferoxamine, an iron chelator, did not affect ROS generation or neurotoxicity. We examined the changes in intracellular copper concentration using a copper-selective fluorescent dye, Phen Green FL, which is quenched by free copper ions. Fluoxetine quenched the fluorescence in neuronal cells, and the quenching effect of fluoxetine was reversed by co-treatment with BCPS, however, not by deferoxamine. These findings demonstrate that fluoxetine could induce apoptotic and oxidative neuronal death associated with an influx of copper ions.
Keyword
MeSH Terms
-
Animals
Antioxidants
Apoptosis
Brain-Derived Neurotrophic Factor
Cell Death
Cell Membrane
Chelating Agents
Chromatin
Copper*
Cycloheximide
Deferoxamine
DNA
Fluorescence
Fluoxetine*
Ions*
Iron
Mice
Neurons*
Reactive Oxygen Species
Serotonin
Antioxidants
Brain-Derived Neurotrophic Factor
Chelating Agents
Chromatin
Copper
Cycloheximide
DNA
Deferoxamine
Fluoxetine
Ions
Iron
Reactive Oxygen Species
Serotonin
Figure
Reference
-
1. Wong DT, Bymaster FP, Engleman EA. Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci. 1995; 57:411–441.
Article2. Pancrazio JJ, Kamatchi GL, Roscoe AK, Lynch C 3rd. Inhibition of neuronal Na+ channels by antidepressant drugs. J Pharmacol Exp Ther. 1998; 284:208–214.3. Deák F, Lasztóczi B, Pacher P, Petheö GL, Kecskeméti V, Spät A. Inhibition of voltage-gated calcium channels by fluoxetine in rat hippocampal pyramidal cells. Neuropharmacology. 2000; 39:1029–1036.
Article4. Ohno Y, Hibino H, Lossin C, Inanobe A, Kurachi Y. Inhibition of astroglial Kir4.1 channels by selective serotonin reuptake inhibitors. Brain Res. 2007; 1178:44–51.
Article5. Nahon E, Israelson A, Abu-Hamad S, Varda SB. Fluoxetine (Prozac) interaction with the mitochondrial voltage-dependent anion channel and protection against apoptotic cell death. FEBS Lett. 2005; 579:5105–5110.
Article6. García-Colunga J, Awad JN, Miledi R. Blockage of muscle and neuronal nicotinic acetylcholine receptors by fluoxetine (Prozac). Proc Natl Acad Sci U S A. 1997; 94:2041–2044.
Article7. Peer D, Dekel Y, Melikhov D, Margalit R. Fluoxetine inhibits multidrug resistance extrusion pumps and enhances responses to chemotherapy in syngeneic and in human xenograft mouse tumor models. Cancer Res. 2004; 64:7562–7569.
Article8. Barygin OI, Nagaeva EI, Tikhonov DB, Belinskaya DA, Vanchakova NP, Shestakova NN. Inhibition of the NMDA and AMPA receptor channels by antidepressants and antipsychotics. Brain Res. 2017; 1660:58–66.
Article9. Brandes LJ, Arron RJ, Bogdanovic RP, Tong J, Zaborniak CL, Hogg GR, et al. Stimulation of malignant growth in rodents by antidepressant drugs at clinically relevant doses. Cancer Res. 1992; 52:3796–3800.10. Spanová A, KovárXMLLink_XYZ H, Lisá V, Lukásová E, Rittich B. Estimation of apoptosis in C6 glioma cells treated with antidepressants. Physiol Res. 1997; 46:161–164.11. Stopper H, Garcia SB, Waaga-Gasser AM, Kannen V. Antidepressant fluoxetine and its potential against colon tumors. World J Gastrointest Oncol. 2014; 6:11–21.
Article12. Serafeim A, Holder MJ, Grafton G, Chamba A, Drayson MT, Luong QT, et al. Selective serotonin reuptake inhibitors directly signal for apoptosis in biopsy-like Burkitt lymphoma cells. Blood. 2003; 101:3212–3219.
Article13. Liu KH, Yang ST, Lin YK, Lin JW, Lee YH, Wang JY, et al. Fluoxetine, an antidepressant, suppresses glioblastoma by evoking AMPAR-mediated calcium-dependent apoptosis. Oncotarget. 2015; 6:5088–5101.
Article14. Kamarudin MNA, Parhar I. Emerging therapeutic potential of anti-psychotic drugs in the management of human glioma: a comprehensive review. Oncotarget. 2019; 10:3952–3977.
Article15. Basso J, Miranda A, Sousa J, Pais A, Vitorino C. Repurposing drugs for glioblastoma: from bench to bedside. Cancer Lett. 2018; 428:173–183.
Article16. Altar CA. Neurotrophins and depression. Trends Pharmacol Sci. 1999; 20:59–61.
Article17. Lee HJ, Kim JW, Yim SV, Kim MJ, Kim SA, Kim YJ, et al. Fluoxetine enhances cell proliferation and prevents apoptosis in dentate gyrus of maternally separated rats. Mol Psychiatry. 2001; 6:610725–728.
Article18. Micheli L, Ceccarelli M, D'Andrea G, Tirone F. Depression and adult neurogenesis: positive effects of the antidepressant fluoxetine and of physical exercise. Brain Res Bull. 2018; 143:181–193.
Article19. Manev R, Uz T, Manev H. Fluoxetine increases the content of neurotrophic protein S100beta in the rat hippocampus. Eur J Pharmacol. 2001; 420:R1–R2.20. Zarei G, Reisi P, Alaei H, Javanmard SH. Effects of amitriptyline and fluoxetine on synaptic plasticity in the dentate gyrus of hippocampal formation in rats. Adv Biomed Res. 2014; 3:199.
Article21. Chiou SH, Chen SJ, Peng CH, Chang YL, Ku HH, Hsu WM, et al. Fluoxetine up-regulates expression of cellular FLICE-inhibitory protein and inhibits LPS-induced apoptosis in hippocampus-derived neural stem cell. Biochem Biophys Res Commun. 2006; 343:391–400.
Article22. Post A, Crochemore C, Uhr M, Holsboer F, Behl C. Differential induction of NF-kappaB activity and neural cell death by antidepressants in vitro. Eur J Neurosci. 2000; 12:4331–4337.
Article23. Levkovitz Y, Gil-Ad I, Zeldich E, Dayag M, Weizman A. Differential induction of apoptosis by antidepressants in glioma and neuroblastoma cell lines: evidence for p-c-Jun, cytochrome c, and caspase-3 involvement. J Mol Neurosci. 2005; 27:29–42.
Article24. Hewett SJ, Csernansky CA, Choi DW. Selective potentiation of NMDA-induced neuronal injury following induction of astrocytic iNOS. Neuron. 1994; 13:487–494.
Article25. Tõugu V, Karafin A, Palumaa P. Binding of zinc(II) and copper(II) to the full-length Alzheimer's amyloid-beta peptide. J Neurochem. 2008; 104:1249–1259.
Article26. Amsterdam JD, Fawcett J, Quitkin FM, Reimherr FW, Rosenbaum JF, Michelson D, et al. Fluoxetine and norfluoxetine plasma concentrations in major depression: a multicenter study. Am J Psychiatry. 1997; 154:963–969.
Article27. Mohindru A, Fisher JM, Rabinovitz M. Bathocuproine sulphonate: a tissue culture-compatible indicator of copper-mediated toxicity. Nature. 1983; 303:64–65.
Article28. Alsop D, Wood CM. Metal and pharmaceutical mixtures: is ion loss the mechanism underlying acute toxicity and widespread additive toxicity in zebrafish? Aquat Toxicol. 2013; 140-141:257–267.
Article29. Castro PA, Ramirez A, Sepúlveda FJ, Peters C, Fierro H, Waldron J, et al. Copper-uptake is critical for the down regulation of synapsin and dynamin induced by neocuproine: modulation of synaptic activity in hippocampal neurons. Front Aging Neurosci. 2014; 6:319.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- N-Acetylcysteine Induces Apoptotic, Oxidative and Excitotoxic Neuronal Death in Mouse Cortical Cultures
- Epilepsy and Oxidative Stress
- Optogenetic and Chemogenetic Approaches for Studying Astrocytes and Gliotransmitters
- The Effects of Agmatine on Apoptosis Induced by Capsaicin in Mouse Hippocampal Neuron
- Epilepsy and Programmed Cell Death