Intest Res.
2019 Oct;17(4):527-536. 10.5217/ir.2019.00031.
Parthenolide inhibits transforming growth factor β1-induced epithelial-mesenchymal transition in colorectal cancer cells
- Affiliations
-
- 1Department of Internal Medicine, Research Institute of Clinical Medicine of Chonbuk National University, and Biomedical Research Institute, Chonbuk National University Hospital, Chonbuk National University Medical School, Jeonju, Korea. clickm@jbnu.ac.kr
- KMID: 2465818
- DOI: http://doi.org/10.5217/ir.2019.00031
Abstract
- BACKGROUND/AIMS
Transforming growth factor-β1 (TGF-β1) induction of epithelial-mesenchymal transition (EMT) is one of the mechanisms by which colorectal cancer (CRC) cells acquire migratory and invasive capacities, and subsequently metastasize. Parthenolide (PT) expresses multiple anti-cancer and anti-inflammatory activities that inhibit nuclear factor κB by targeting the IκB kinase complex. In the present study, we aimed to investigate whether PT can inhibit TGF-β1-induced EMT in CRC cell lines.
METHODS
HT-29 and SW480 cell lines were used in the experiment. Cell viability was detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and sub-G1 analysis was measured by flow cytometry. The induction of EMT by TGF-β1 and inhibition of the process by PT was analyzed by phase contrast microscopy, wounding healing, cellular migration and invasion assays, and Western blotting.
RESULTS
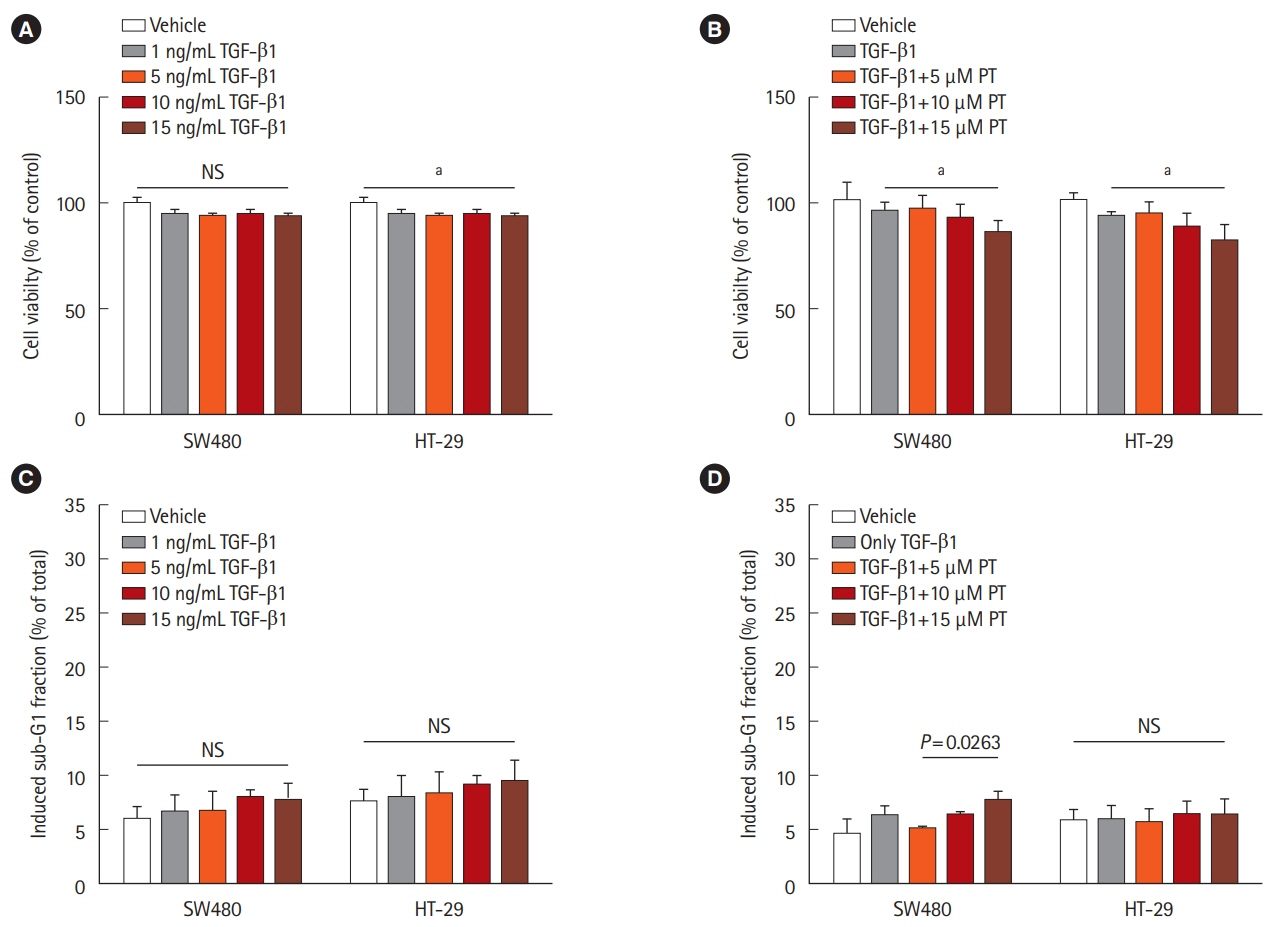
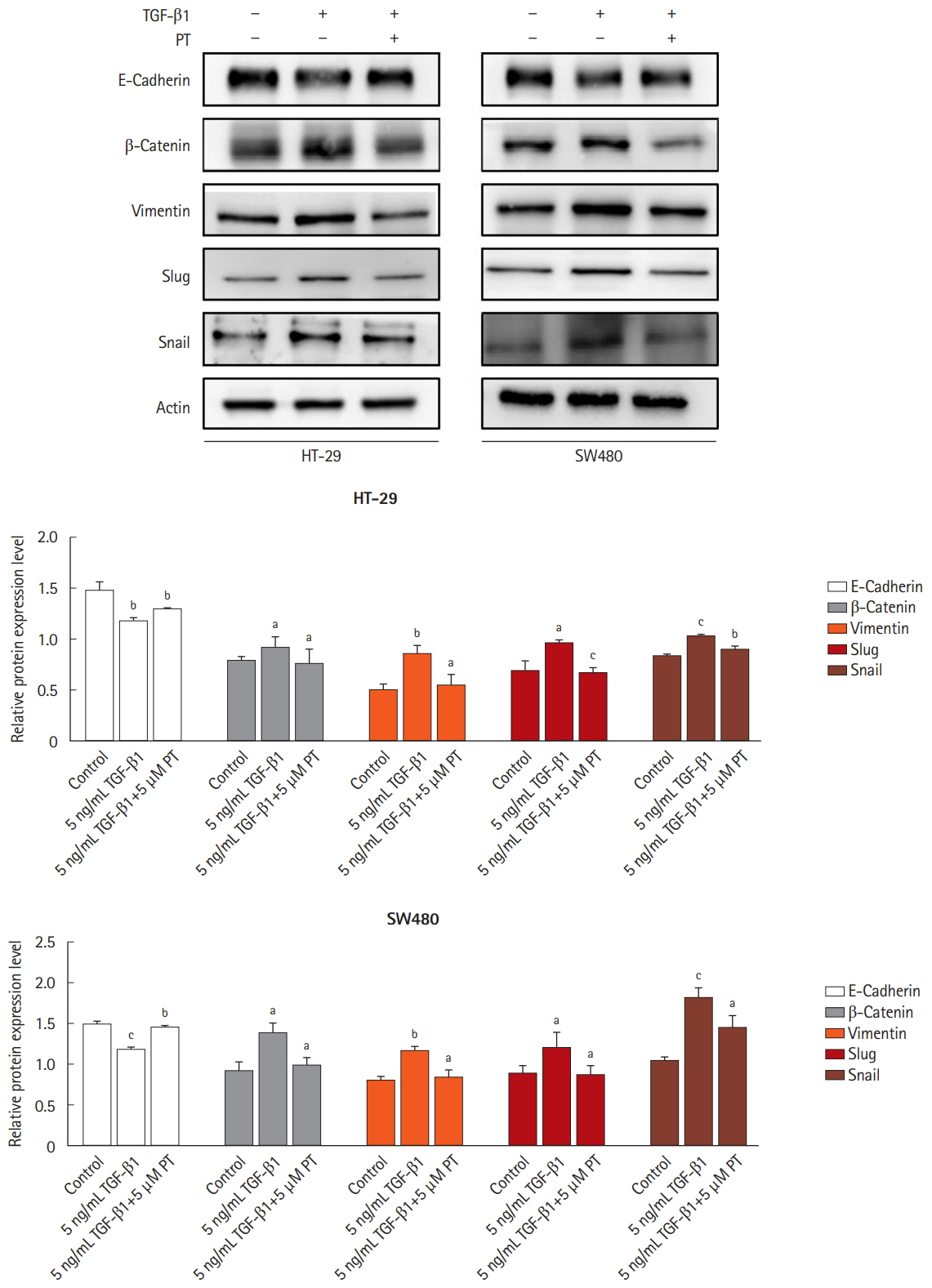
TGF-β1 inhibits HT-29 cell proliferation, but has no effect on SW480 cell proliferation; different concentrations of TGF-β1 did not induce apoptosis in HT-29 and SW480 cells. PT attenuates TGF-β1-induced elongated, fibroblast-like shape changing in cells. PT inhibits TGF-β1-induced cell migration and cell invasion. In addition, other EMT markers such as β-catenin, Vimentin, Snail, and Slug were suppressed by PT, while E-cadherin was increased by PT.
CONCLUSIONS
Our findings show that PT inhibits TGF-β1-induced EMT by suppressing the expression of the mesenchymal protein and increasing expression of the epithelial protein. These findings suggest a novel approach for CRC treatment by suppression of TGF-β1-induced EMT.
Keyword
MeSH Terms
-
Apoptosis
Blotting, Western
Cadherins
Cell Line
Cell Movement
Cell Proliferation
Cell Survival
Colorectal Neoplasms*
Epithelial-Mesenchymal Transition*
Flow Cytometry
Gastropoda
HT29 Cells
Humans
Microscopy, Phase-Contrast
Phosphotransferases
Snails
Transforming Growth Factors*
Vimentin
Wounds and Injuries
Cadherins
Phosphotransferases
Transforming Growth Factors
Vimentin
Figure
Reference
-
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018; 68:7–30.
Article2. Raval M, Bande D, Pillai AK, et al. Yttrium-90 radioembolization of hepatic metastases from colorectal cancer. Front Oncol. 2014; 4:120.3. Villalba M, Evans SR, Vidal-Vanaclocha F, Calvo A. Role of TGF-beta in metastatic colon cancer: it is finally time for targeted therapy. Cell Tissue Res. 2017; 370:29–39.4. Gonzalez-Zubeldia I, Dotor J, Redrado M, et al. Co-migration of colon cancer cells and CAFs induced by TGFbeta1 enhances liver metastasis. Cell Tissue Res. 2015; 359:829–839.5. Massagué J. TGF-beta signal transduction. Annu Rev Biochem. 1998; 67:753–791.6. Lampropoulos P, Zizi-Sermpetzoglou A, Rizos S, Kostakis A, Nikiteas N, Papavassiliou AG. TGF-beta signalling in colon carcinogenesis. Cancer Lett. 2012; 314:1–7.7. Bremnes RM, Dønnem T, Al-Saad S, et al. The role of tumor stroma in cancer progression and prognosis: emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J Thorac Oncol. 2011; 6:209–217.8. Padua D, Massagué J. Roles of TGFbeta in metastasis. Cell Res. 2009; 19:89–102.9. Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001; 29:117–129.10. Akhurst RJ, Derynck R. TGF-beta signaling in cancer: a double-edged sword. Trends Cell Biol. 2001; 11:S44–S51.11. Lim J, Thiery JP. Epithelial-mesenchymal transitions: insights from development. Development. 2012; 139:3471–3486.12. Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol. 2012; 22:396–403.
Article13. Peñuelas S, Anido J, Prieto-Sánchez RM, et al. TGF-beta increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell. 2009; 15:315–327.14. Massagué J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012; 13:616–630.15. Jafari N, Nazeri S, Enferadi ST. Parthenolide reduces metastasis by inhibition of vimentin expression and induces apoptosis by suppression elongation factor alpha-1 expression. Phytomedicine. 2018; 41:67–73.16. Fu J, Ke X, Tan S, et al. The natural compound codonolactone attenuates TGF-beta1-mediated epithelial-to-mesenchymal transition and motility of breast cancer cells. Oncol Rep. 2016; 35:117–126.
Article17. Chiu KY, Wu CC, Chia CH, Hsu SL, Tzeng YM. Inhibition of growth, migration and invasion of human bladder cancer cells by antrocin, a sesquiterpene lactone isolated from Antrodia cinnamomea, and its molecular mechanisms. Cancer Lett. 2016; 373:174–184.18. Liu JW, Cai MX, Xin Y, et al. Parthenolide induces proliferation inhibition and apoptosis of pancreatic cancer cells in vitro. J Exp Clin Cancer Res. 2010; 29:108.
Article19. Liu YC, Kim SL, Park YR, Lee ST, Kim SW. Parthenolide promotes apoptotic cell death and inhibits the migration and invasion of SW620 cells. Intest Res. 2017; 15:174–181.20. Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. 2005; 17:559–564.
Article21. Natalwala A, Spychal R, Tselepis C. Epithelial-mesenchymal transition mediated tumourigenesis in the gastrointestinal tract. World J Gastroenterol. 2008; 14:3792–3797.
Article22. Massagué J. TGFbeta in cancer. Cell. 2008; 134:215–230.23. Woodford-Richens KL, Rowan AJ, Gorman P, et al. SMAD4 mutations in colorectal cancer probably occur before chromosomal instability, but after divergence of the microsatellite instability pathway. Proc Natl Acad Sci U S A. 2001; 98:9719–9723.
Article24. Jung B, Staudacher JJ, Beauchamp D. Transforming growth factor beta superfamily signaling in development of colorectal cancer. Gastroenterology. 2017; 152:36–52.25. Levy L, Hill CS. Smad4 dependency defines two classes of transforming growth factor {beta} (TGF-{beta}) target genes and distinguishes TGF-{beta}-induced epithelial-mesenchymal transition from its antiproliferative and migratory responses. Mol Cell Biol. 2005; 25:8108–8125.
Article26. Jones S, Chen WD, Parmigiani G, et al. Comparative lesion sequencing provides insights into tumor evolution. Proc Natl Acad Sci U S A. 2008; 105:4283–4288.
Article27. Alazzouzi H, Alhopuro P, Salovaara R, et al. SMAD4 as a prognostic marker in colorectal cancer. Clin Cancer Res. 2005; 11:2606–2611.
Article28. Miyaki M, Iijima T, Konishi M, et al. Higher frequency of Smad4 gene mutation in human colorectal cancer with distant metastasis. Oncogene. 1999; 18:3098–3103.
Article29. Zhang B, Halder SK, Kashikar ND, et al. Antimetastatic role of Smad4 signaling in colorectal cancer. Gastroenterology. 2010; 138:969–980.30. Pino MS, Kikuchi H, Zeng M, et al. Epithelial to mesenchymal transition is impaired in colon cancer cells with microsatellite instability. Gastroenterology. 2010; 138:1406–1417.
Article31. Vu T, Datta PK. Regulation of EMT in colorectal cancer: a culprit in metastasis. Cancers (Basel). 2017; 9:E171.
Article32. Tian X, Liu Z, Niu B, et al. E-cadherin/beta-catenin complex and the epithelial barrier. J Biomed Biotechnol. 2011; 2011:567305.33. Kim SL, Park YR, Lee ST, Kim SW. Parthenolide suppresses hypoxia-inducible factor-1alpha signaling and hypoxia induced epithelial-mesenchymal transition in colorectal cancer. Int J Oncol. 2017; 51:1809–1820.
Article34. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009; 119:1420–1428.
Article35. Huber MA, Azoitei N, Baumann B, et al. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004; 114:569–581.36. Grau AM, Datta PK, Zi J, Halder SK, Beauchamp RD. Role of Smad proteins in the regulation of NF-kappaB by TGF-beta in colon cancer cells. Cell Signal. 2006; 18:1041–1050.
Article37. Zhao L, Liu S, Che X, et al. Bufalin inhibits TGF-beta-induced epithelial-to-mesenchymal transition and migration in human lung cancer A549 cells by downregulating TGF-beta receptors. Int J Mol Med. 2015; 36:645–652.
Article38. Xu Y, Lou Z, Lee SH. Arctigenin represses TGF-beta-induced epithelial mesenchymal transition in human lung cancer cells. Biochem Biophys Res Commun. 2017; 493:934–939.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dexamethasone Inhibits TGF-β1-Induced Cell Migration by Regulating the ERK and AKT Pathways in Human Colon Cancer Cells Via CYR61
- Apolipoprotein A1 Inhibits TGF-β1-Induced Epithelial-to-Mesenchymal Transition of Alveolar Epithelial Cells
- Trefoil Factor 1 Suppresses Epithelial-mesenchymal Transition through Inhibition of TGF-beta Signaling in Gastric Cancer Cells
- Aspirin-Triggered Resolvin D1 Inhibits TGF-β1-Induced EndMT through Increasing the Expression of Smad7 and Is Closely Related to Oxidative Stress
- Tumor-Associated Macrophages Derived TGF-β‒Induced Epithelial to Mesenchymal Transition in Colorectal Cancer Cells through Smad2,3-4/Snail Signaling Pathway