J Lipid Atheroscler.
2019 Sep;8(2):267-276. 10.12997/jla.2019.8.2.267.
Anti-malarial Drugs Reduce Vascular Smooth Muscle Cell Proliferation via Activation of AMPK and Inhibition of Smad3 Signaling
- Affiliations
-
- 1Department of Pharmacology and Smart-Ageing Convergence Research Center, Yeungnam University College of Medicine, Daegu, Korea. changhoon_woo@yu.ac.kr
- KMID: 2458392
- DOI: http://doi.org/10.12997/jla.2019.8.2.267
Abstract
OBJECTIVE
The aim of this study was to investigate the effects of 2 anti-malarial drugs, chloroquine (CQ) and hydroxychloroquine (HCQ), on inhibition of vascular smooth muscle cell (VSMC) proliferation both in vivo and in vitro via Adenosine monophosphate-activated protein kinase (AMPK) activation.
METHODS
Protein and mRNA levels were determined by western blot analysis and real-time reverse transcription-polymerase chain reaction in primary rat VSMCs treated with CQ and HCQ, respectively. Cell proliferation was measured by flow cytometry and cell counting. Mice carotid arteries were ligated and treated with CQ or HCQ every other day for 3 weeks. Pathological changes of carotid arteries were visualized by both microscopy and fluorescence microscopy.
RESULTS
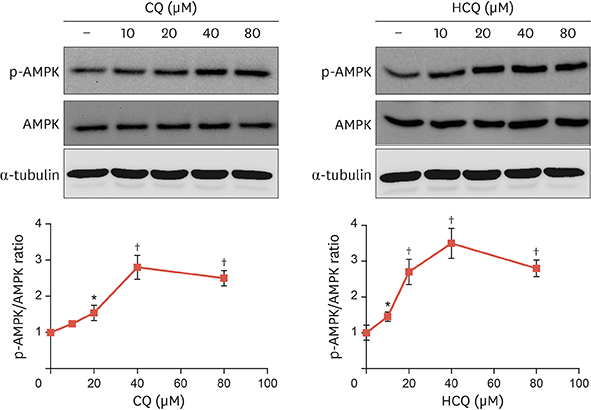
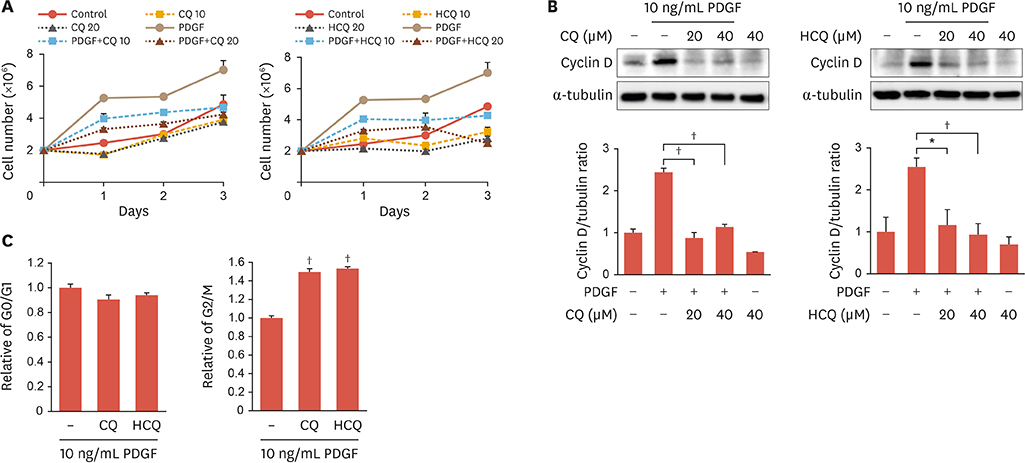
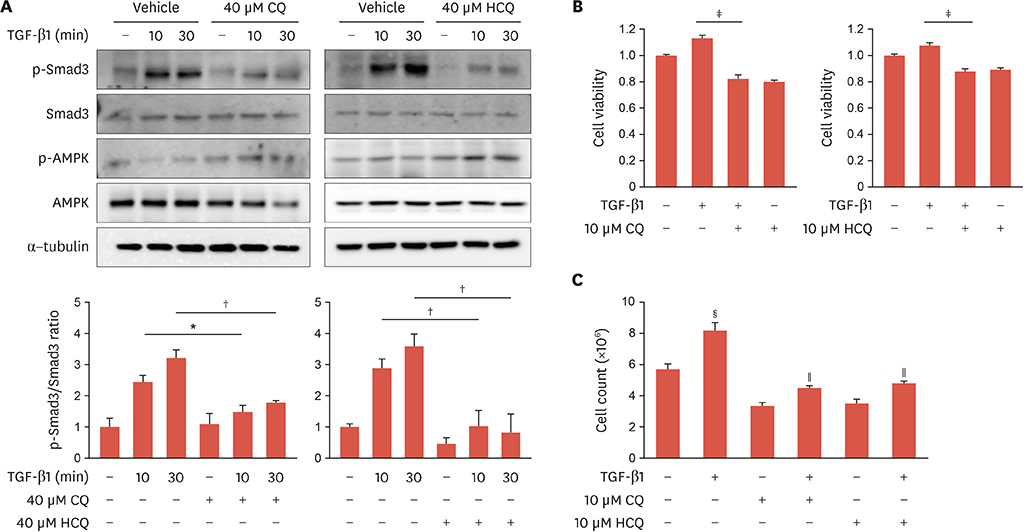
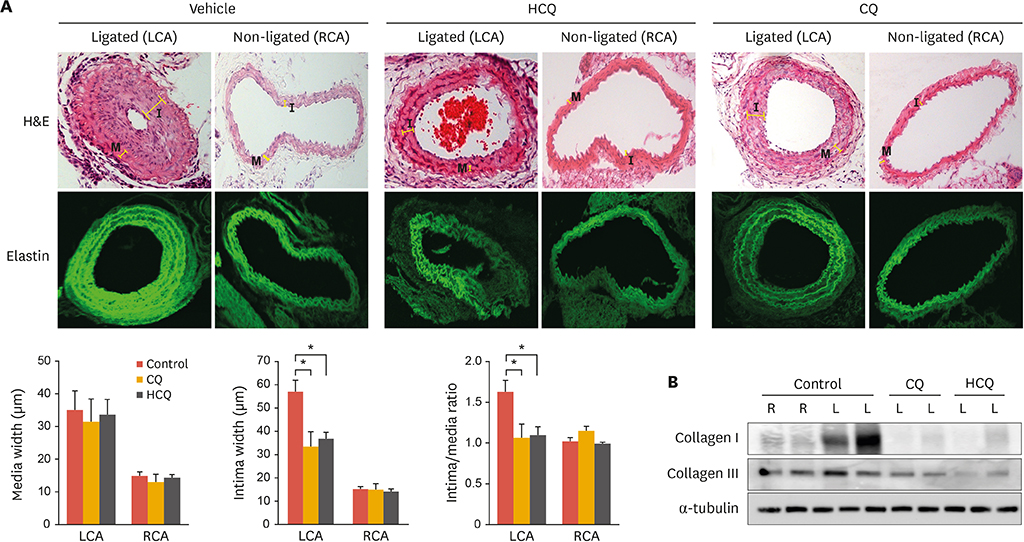
CQ and HCQ increase AMPK phosphorylation in VSMCs. Both CQ and HCQ decrease platelet-derived growth factor-induced VSMC proliferation and cell cycle progression in an AMPK-dependent manner. In addition, CQ and HCQ inhibit Smad3 phosphorylation and VSMC proliferation induced by transforming growth factor-β1. Moreover, CQ and HCQ diminished neointimal proliferation in a mouse model of carotid artery ligation-induced neointima formation.
CONCLUSION
The results demonstrated that CQ and HCQ inhibit cell proliferation and cell cycle progression in VSMCs via the AMPK-dependent signaling pathway. Carotid artery ligation-induced intima thickness was reduced in mouse arteries treated with CQ or HCQ, suggesting a role for antimalarial drugs in treating atherosclerosis and restenosis.
Keyword
MeSH Terms
-
Adenosine
AMP-Activated Protein Kinases*
Animals
Antimalarials
Arteries
Atherosclerosis
Blotting, Western
Carotid Arteries
Cell Count
Cell Cycle
Cell Proliferation*
Chloroquine
Flow Cytometry
Hydroxychloroquine
In Vitro Techniques
Mice
Microscopy
Microscopy, Fluorescence
Muscle, Smooth, Vascular*
Neointima
Phosphorylation
Protein Kinases
Rats
RNA, Messenger
AMP-Activated Protein Kinases
Adenosine
Antimalarials
Chloroquine
Hydroxychloroquine
Protein Kinases
RNA, Messenger
Figure
Reference
-
1. Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004; 84:767–801.
Article2. Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016; 118:692–702.
Article3. Durham AL, Speer MY, Scatena M, Giachelli CM, Shanahan CM. Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc Res. 2018; 114:590–600.
Article4. Fuster JJ, Fernández P, González-Navarro H, Silvestre C, Nabah YN, Andrés V. Control of cell proliferation in atherosclerosis: insights from animal models and human studies. Cardiovasc Res. 2010; 86:254–264.
Article5. Rudijanto A. The role of vascular smooth muscle cells on the pathogenesis of atherosclerosis. Acta Med Indones. 2007; 39:86–93.6. Rensen SS, Doevendans PA, van Eys GJ. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J. 2007; 15:100–108.
Article7. Golden EB, Cho HY, Hofman FM, Louie SG, Schönthal AH, Chen TC. Quinoline-based antimalarial drugs: a novel class of autophagy inhibitors. Neurosurg Focus. 2015; 38:E12.
Article8. Mauthe M, Orhon I, Rocchi C, Zhou X, Luhr M, Hijlkema KJ, et al. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy. 2018; 14:1435–1455.
Article9. Solomon VR, Lee H. Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur J Pharmacol. 2009; 625:220–233.
Article10. Lee SJ, Silverman E, Bargman JM. The role of antimalarial agents in the treatment of SLE and lupus nephritis. Nat Rev Nephrol. 2011; 7:718–729.
Article11. Rynes RI. Antimalarial drugs in the treatment of rheumatological diseases. 1997; 36:799–805.
Article12. Sharma TS, Wasko MC, Tang X, Vedamurthy D, Yan X, Cote J, et al. Hydroxychloroquine use is associated with decreased incident cardiovascular events in rheumatoid arthritis patients. J Am Heart Assoc. 2016; 5:e002867.
Article13. Long L, Yang X, Southwood M, Lu J, Marciniak SJ, Dunmore BJ, et al. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ Res. 2013; 112:1159–1170.
Article14. Chen D, Xie J, Fiskesund R, Dong W, Liang X, Lv J, et al. Chloroquine modulates antitumor immune response by resetting tumor-associated macrophages toward M1 phenotype. Nat Commun. 2018; 9:873.
Article15. Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011; 13:1016–1023.
Article16. Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007; 403:139–148.
Article17. Rubin LJ, Magliola L, Feng X, Jones AW, Hale CC. Metabolic activation of AMP kinase in vascular smooth muscle. J Appl Physiol (1985). 2005; 98:296–306.
Article18. Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation--AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol. 2006; 574:63–71.
Article19. Stone JD, Narine A, Shaver PR, Fox JC, Vuncannon JR, Tulis DA. AMP-activated protein kinase inhibits vascular smooth muscle cell proliferation and migration and vascular remodeling following injury. Am J Physiol Heart Circ Physiol. 2013; 304:H369–H381.
Article20. Stone JD, Narine A, Tulis DA. Inhibition of vascular smooth muscle growth via signaling crosstalk between AMP-activated protein kinase and cAMP-dependent protein kinase. Front Physiol. 2012; 3:409.
Article21. Zou MH, Wu Y. AMP-activated protein kinase activation as a strategy for protecting vascular endothelial function. Clin Exp Pharmacol Physiol. 2008; 35:535–545.
Article22. Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-β signaling in fibrosis. Growth Factors. 2011; 29:196–202.
Article23. Pardali E, Ten Dijke P. TGFβ signaling and cardiovascular diseases. Int J Biol Sci. 2012; 8:195–213.
Article24. Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov. 2012; 11:790–811.
Article25. Hneino M, Bouazza L, Bricca G, Li JY, Langlois D. Density-dependent shift of transforming growth factor-beta-1 from inhibition to stimulation of vascular smooth muscle cell growth is based on unconventional regulation of proliferation, apoptosis and contact inhibition. J Vasc Res. 2009; 46:85–97.
Article26. Samarakoon R, Higgins SP, Higgins CE, Higgins PJ. TGF-beta1-induced plasminogen activator inhibitor-1 expression in vascular smooth muscle cells requires pp60(c-src)/EGFR(Y845) and Rho/ROCK signaling. J Mol Cell Cardiol. 2008; 44:527–538.
Article27. Suwanabol PA, Seedial SM, Shi X, Zhang F, Yamanouchi D, Roenneburg D, et al. Transforming growth factor-β increases vascular smooth muscle cell proliferation through the Smad3 and extracellular signal-regulated kinase mitogen-activated protein kinases pathways. J Vasc Surg. 2012; 56:446–454.
Article28. Tsai S, Hollenbeck ST, Ryer EJ, Edlin R, Yamanouchi D, Kundi R, et al. TGF-beta through Smad3 signaling stimulates vascular smooth muscle cell proliferation and neointimal formation. Am J Physiol Heart Circ Physiol. 2009; 297:H540–H549.29. Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, et al. A model of disturbed flow-induced atherosclerosis in mouse carotid artery by partial ligation and a simple method of RNA isolation from carotid endothelium. J Vis Exp. 2010; 1861.
Article30. Stone JD, Holt AW, Vuncannon JR, Brault JJ, Tulis DA. AMP-activated protein kinase inhibits transforming growth factor-β-mediated vascular smooth muscle cell growth: implications for a Smad-3-dependent mechanism. Am J Physiol Heart Circ Physiol. 2015; 309:H1251–H1259.
Article31. Sperber K, Quraishi H, Kalb TH, Panja A, Stecher V, Mayer L. Selective regulation of cytokine secretion by hydroxychloroquine: inhibition of interleukin 1 alpha (IL-1-alpha) and IL-6 in human monocytes and T cells. J Rheumatol. 1993; 20:803–808.32. Wozniacka A, Lesiak A, Narbutt J, McCauliffe DP, Sysa-Jedrzejowska A. Chloroquine treatment influences proinflammatory cytokine levels in systemic lupus erythematosus patients. Lupus. 2006; 15:268–275.
Article33. Fan C, Wang W, Zhao B, Zhang S, Miao J. Chloroquine inhibits cell growth and induces cell death in A549 lung cancer cells. Bioorg Med Chem. 2006; 14:3218–3222.
Article34. Zheng Y, Zhao YL, Deng X, Yang S, Mao Y, Li Z, et al. Chloroquine inhibits colon cancer cell growth in vitro and tumor growth in vivo via induction of apoptosis. Cancer Invest. 2009; 27:286–292.
Article35. Monma H, Iida Y, Moritani T, Okimoto T, Tanino R, Tajima Y, et al. Chloroquine augments TRAIL-induced apoptosis and induces G2/M phase arrest in human pancreatic cancer cells. PLoS One. 2018; 13:e0193990.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Losartan Inhibits Vascular Smooth Muscle Cell Proliferation through Activation of AMP-Activated Protein Kinase
- Mesoglycan attenuates VSMC proliferation through activation of AMP-activated protein kinase and mTOR
- Cilostazol Inhibits Vascular Smooth Muscle Cell Proliferation and Reactive Oxygen Species Production through Activation of AMP-activated Protein Kinase Induced by Heme Oxygenase-1
- The effect of anti-hypertensive drugs on DNA synthesis and proliferation of ultured rat aortic smooth muscle cells
- Zingerone Attenuates Pi-induced Vascular Calcification via AMPK-mediated TIMP4 Expression