Korean J Physiol Pharmacol.
2011 Aug;15(4):203-210. 10.4196/kjpp.2011.15.4.203.
Cilostazol Inhibits Vascular Smooth Muscle Cell Proliferation and Reactive Oxygen Species Production through Activation of AMP-activated Protein Kinase Induced by Heme Oxygenase-1
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Yeungnam University, Daegu 705-717, Korea. hcchoi@med.yu.ac.kr
- 2Aging-associated Vascular Disease Research Center, College of Medicine, Yeungnam University, Daegu 705-717, Korea.
- 3Department of Microbiology, College of Medicine, Yeungnam University, Daegu 705-717, Korea.
- 4Department of Surgery, College of Medicine, Yeungnam University, Daegu 705-717, Korea.
- KMID: 1448925
- DOI: http://doi.org/10.4196/kjpp.2011.15.4.203
Abstract
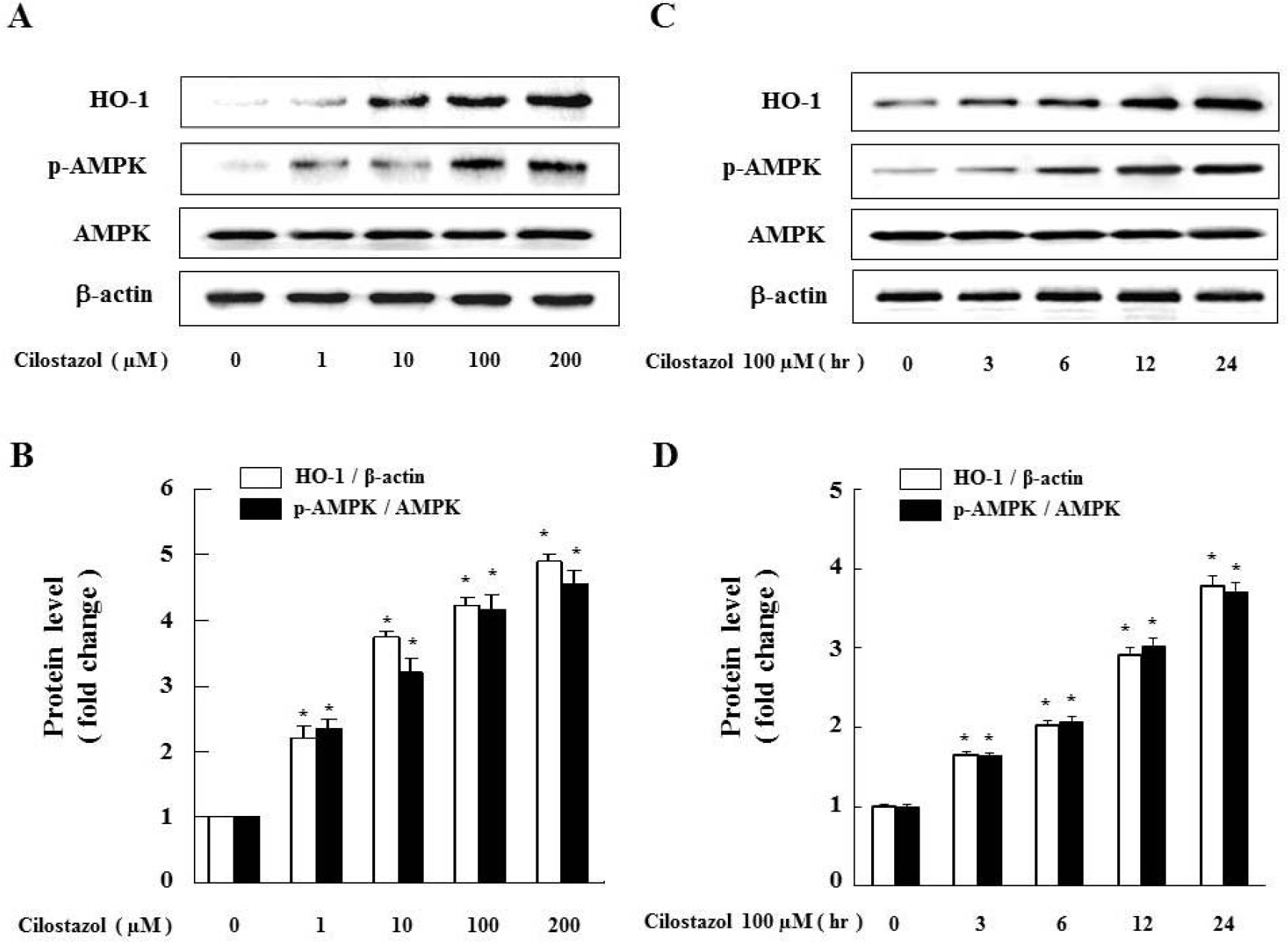
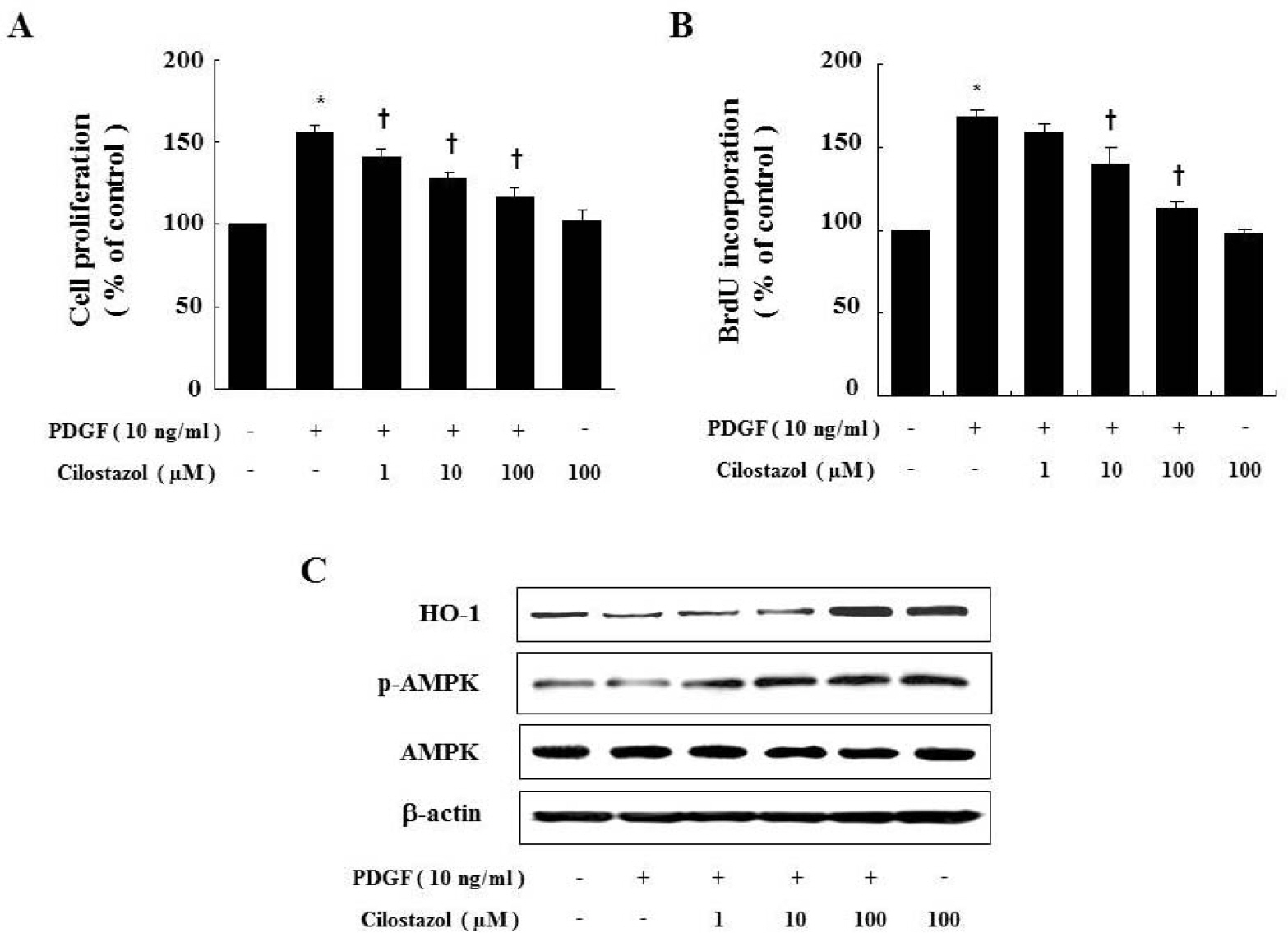
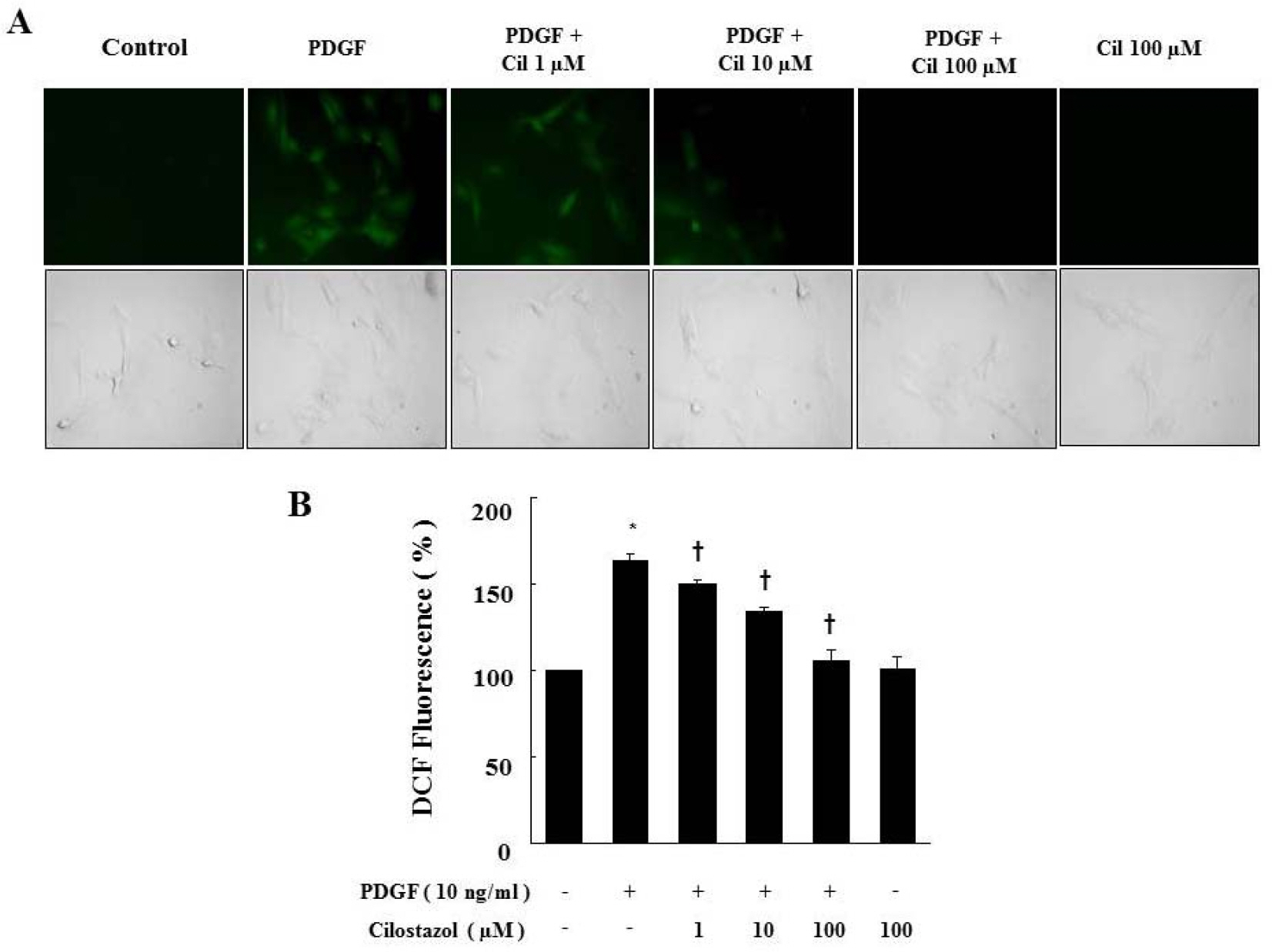
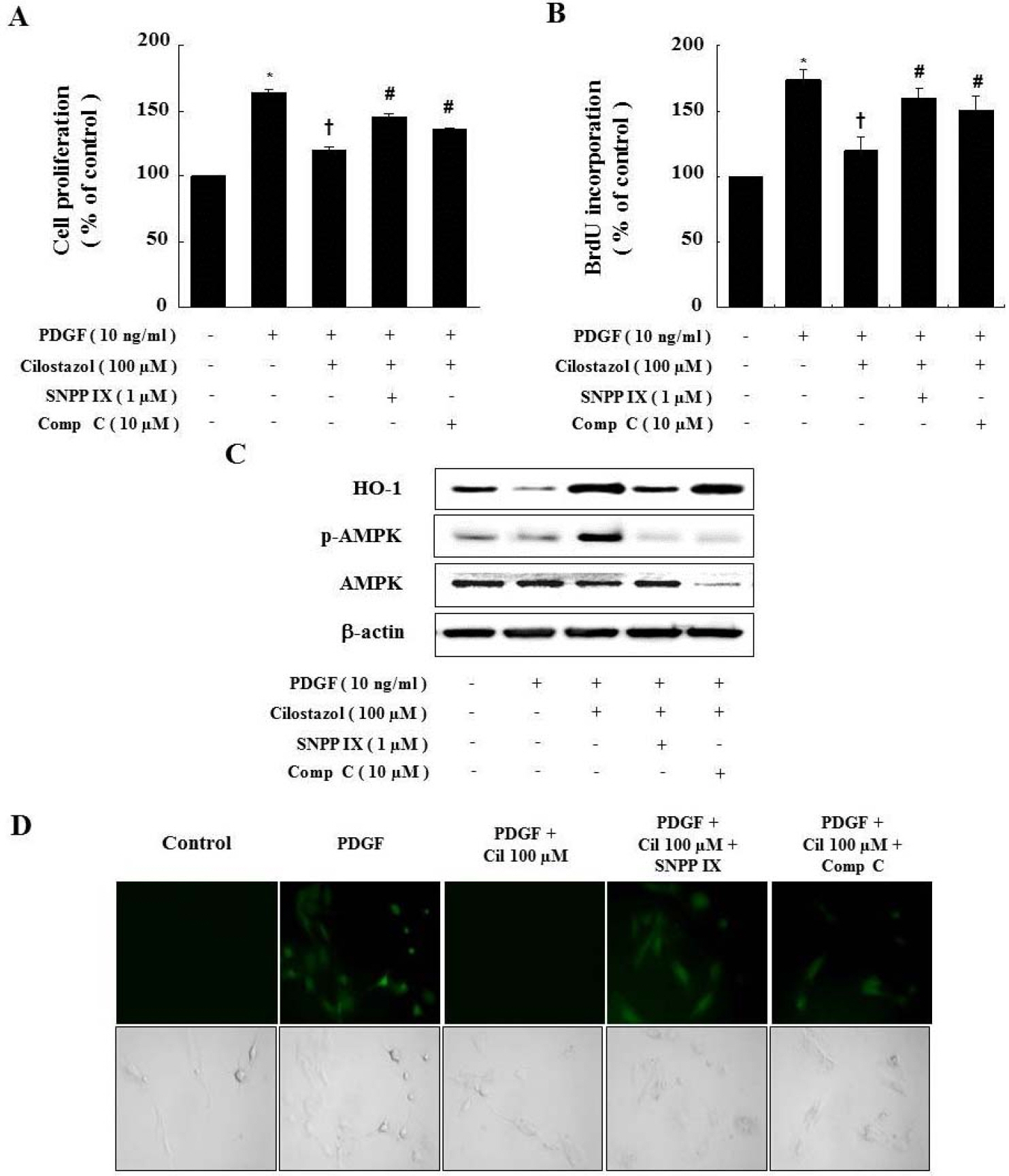
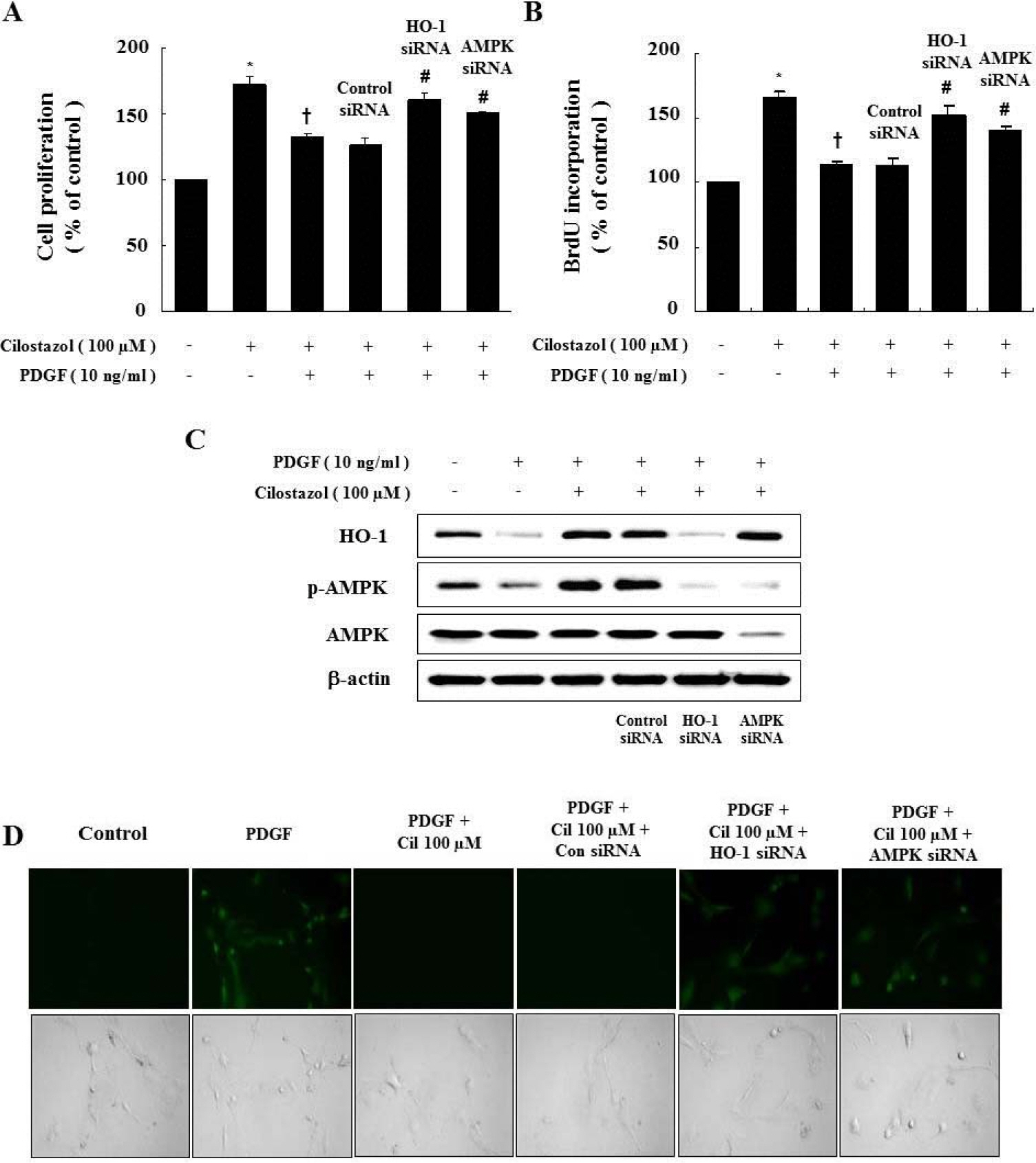
- Cilostazol is a selective inhibitor of phosphodiesterase 3 that increases intracellular cAMP levels and activates protein kinase A, thereby inhibiting vascular smooth muscle cell (VSMC) proliferation. We investigated whether AMP-activated protein kinase (AMPK) activation induced by heme oxygenase-1 (HO-1) is a mediator of the beneficial effects of cilostazol and whether cilostazol may prevent cell proliferation and reactive oxygen species (ROS) production by activating AMPK in VSMC. In the present study, we investigated VSMC with various concentrations of cilostazol. Treatment with cilostazol increased HO-1 expression and phosphorylation of AMPK in a dose- and time-dependent manner. Cilostazol also significantly decreased platelet-derived growth factor (PDGF)-induced VSMC proliferation and ROS production by activating AMPK induced by HO-1. Pharmacological and genetic inhibition of HO-1 and AMPK blocked the cilostazol-induced inhibition of cell proliferation and ROS production.These data suggest that cilostazol-induced HO-1 expression and AMPK activation might attenuate PDGF-induced VSMC proliferation and ROS production.
Keyword
MeSH Terms
-
AMP-Activated Protein Kinases
Cell Proliferation
Cyclic AMP-Dependent Protein Kinases
Heme
Heme Oxygenase-1
Muscle, Smooth, Vascular
Phosphorylation
Platelet-Derived Growth Factor
Reactive Oxygen Species
Tetrazoles
AMP-Activated Protein Kinases
Cyclic AMP-Dependent Protein Kinases
Heme
Heme Oxygenase-1
Platelet-Derived Growth Factor
Reactive Oxygen Species
Tetrazoles
Figure
Cited by 1 articles
-
Regulation of Ca2+ Signaling in Pulmonary Hypertension
Amy L. Firth, Jun Yeon Won, Won Sun Park
Korean J Physiol Pharmacol. 2013;17(1):1-8. doi: 10.4196/kjpp.2013.17.1.1.
Reference
-
References
1. Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004; 84:767–801.
Article2. Zhang C. MicroRNA-145 in vascular smooth muscle cell biology: a new therapeutic target for vascular disease. Cell Cycle. 2009; 8:3469–3473.
Article3. Ruygrok PN, Muller DW, Serruys PW. Rapamycin in cardiovascular medicine. Intern Med J. 2003; 33:103–109.
Article4. Braun-Dullaeus RC, Mann MJ, Dzau VJ. Cell cycle progression: new therapeutic target for vascular proliferative disease. Circulation. 1998; 98:82–89.5. Yang X, Thomas DP, Zhang X, Culver BW, Alexander BM, Murdoch WJ, Rao MN, Tulis DA, Ren J, Sreejayan N. Curcumin inhibits platelet-derived growth factor-stimulated vascular smooth muscle cell function and injury-induced neointima formation. Arterioscler Thromb Vasc Biol. 2006; 26:85–90.
Article6. Park J, Ha H, Seo J, Kim MS, Kim HJ, Huh KH, Park K, Kim YS. Mycophenolic acid inhibits platelet-derived growth factor-induced reactive oxygen species and mitogen-activated protein kinase activation in rat vascular smooth muscle cells. Am J Transplant. 2004; 4:1982–1990.
Article7. Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006; 71:247–258.
Article8. Mesquita FS, Dyer SN, Heinrich DA, Bulun SE, Marsh EE, Nowak RA. Reactive oxygen species mediate mitogenic growth factor signaling pathways in human leiomyoma smooth muscle cells. Biol Reprod. 2010; 82:341–351.9. Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003; 546:113–120.
Article10. Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997; 273:E1107–1112.11. Abraham NG, Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Radic Biol Med. 2005; 39:1–25.
Article12. Stocker R, Perrella MA. Heme oxygenase-1: a novel drug target for atherosclerotic diseases? Circulation. 2006; 114:2178–2189.13. Durante W, Christodoulides N, Cheng K, Peyton KJ, Sunahara RK, Schafer AI. cAMP induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle. Am J Physiol. 1997; 273:H317–323.
Article14. Kambayashi J, Liu Y, Sun B, Shakur Y, Yoshitake M, Czerwiec F. Cilostazol as a unique antithrombotic agent. Curr Pharm Des. 2003; 9:2289–2302.
Article15. Dawson DL, Cutler BS, Meissner MH, Strandness DE Jr. Cilostazol has beneficial effects in treatment of intermittent claudication: results from a multicenter, randomized, prospective, double-blind trial. Circulation. 1998; 98:678–686.
Article16. Stone WM, Demaerschalk BM, Fowl RJ, Money SR. Type 3 phosphodiesterase inhibitors may be protective against cerebrovascular events in patients with claudication. J Stroke Cerebrovasc Dis. 2008; 17:129–133.
Article17. Kim MJ, Park KG, Lee KM, Kim HS, Kim SY, Kim CS, Lee SL, Chang YC, Park JY, Lee KU, Lee IK. Cilostazol inhibits vascular smooth muscle cell growth by downregulation of the transcription factor E2F. Hypertension. 2005; 45:552–556.
Article18. Ryter SW, Choi AM. Heme oxygenase-1: redox regulation of a stress protein in lung and cell culture models. Antioxid Redox Signal. 2005; 7:80–91.
Article19. Choi HC, Lee KY, Lee DH, Kang YJ. Heme oxygenase-1 induced by aprotinin inhibits vascular smooth muscle cell proliferation through cell cycle arrest in hypertensive rats. Korean J Physiol Pharmacol. 2009; 13:309–313.
Article20. Immenschuh S, Hinke V, Ohlmann A, Gifhorn-Katz S, Katz N, Jungermann K, Kietzmann T. Transcriptional activation of the haem oxygenase-1 gene by cGMP via a cAMP response element/activator protein-1 element in primary cultures of rat hepatocytes. Biochem J. 1998; 334:141–146.
Article21. L'Abbate A, Neglia D, Vecoli C, Novelli M, Ottaviano V, Baldi S, Barsacchi R, Paolicchi A, Masiello P, Drummond GS, McClung JA, Abraham NG. Beneficial effect of heme oxygenase-1 expression on myocardial ischemia-reperfusion involves an increase in adiponectin in mildly diabetic rats. Am J Physiol Heart Circ Physiol. 2007; 293:H3532–3541.22. Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002; 8:1288–1295.
Article23. Hardie DG. The AMP-activated protein kinase pathway–new players upstream and downstream. J Cell Sci. 2004; 117:5479–5487.24. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001; 108:1167–1174.
Article25. Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002; 8:1288–1295.
Article26. Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation-AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol. 2006; 574:63–71.27. Sung JY, Choi HC. Aspirin-induced AMP-activated protein kinase activation regulates the proliferation of vascular smooth muscle cells from spontaneously hypertensive rats. Biochem Biophys Res Commun. 2011; 408:312–317.
Article28. Kim MJ, Lee JH, Park SY, Hong KW, Kim CD, Kim KY, Lee WS. Protection from apoptotic cell death by cilostazol, phosphodiesterase type III inhibitor, via cAMP-dependent protein kinase activation. Pharmacol Res. 2006; 54:261–267.
Article29. Merry TL, Steinberg GR, Lynch GS, McConell GK. Skeletal muscle glucose uptake during contraction is regulated by nitric oxide and ROS independently of AMPK. Am J Physiol Endocrinol Metab. 2010; 298:E577–585.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fraxetin Induces Heme Oxygenase-1 Expression by Activation of Akt/Nrf2 or AMP-activated Protein Kinase α/Nrf2 Pathway in HaCaT Cells
- Losartan Inhibits Vascular Smooth Muscle Cell Proliferation through Activation of AMP-Activated Protein Kinase
- 5-aminoimidazole-4-carboxamide Riboside Induces Apoptosis Through AMP-activated Protein Kinase-independent and NADPH Oxidase-dependent Pathways
- Heme Oxygenase-1 Induced by Aprotinin Inhibits Vascular Smooth Muscle Cell Proliferation Through Cell Cycle Arrest in Hypertensive Rats
- Lonchocarpine Increases Nrf2/ARE-Mediated Antioxidant Enzyme Expression by Modulating AMPK and MAPK Signaling in Brain Astrocytes