Anat Cell Biol.
2017 Jun;50(2):107-114. 10.5115/acb.2017.50.2.107.
Condition medium of cerebrospinal fluid and retinoic acid induces the transdifferentiation of human dental pulp stem cells into neuroglia and neural like cells
- Affiliations
-
- 1Department of Anatomy and Cell Biology, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran. h.ghasemi@mazums.ac.ir
- 2Department of Anatomy and Cell Biology, Molecular and Cell Biology Research Center, Mazandaran University of Medical Sciences, Sari, Iran.
- 3Cellular and Molecular Research Center, Qazvin University of Medical Science, Qazvin, Iran.
- 4Department of Neurology, School of Medicine, Immunogenetic Research Center, Clinical Research Development Unit of Bou Ali Sina Hospital, Mazandaran University of Medical Sciences, Sari, Iran.
- 5Department of Basic Sciences, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
- 6Department of Anatomy and Cell Biology, Immunogenetic Research Center, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
- 7Department of Medical Genetics, Faculty of Medicine & Dentistry, University of Alberta, Edmonton, Alberta, Canada.
- KMID: 2451236
- DOI: http://doi.org/10.5115/acb.2017.50.2.107
Abstract
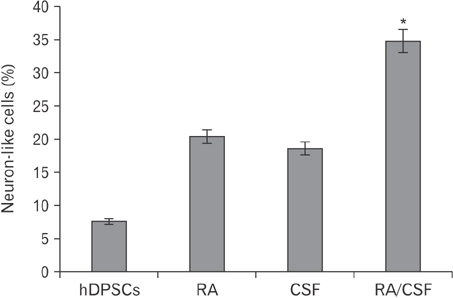
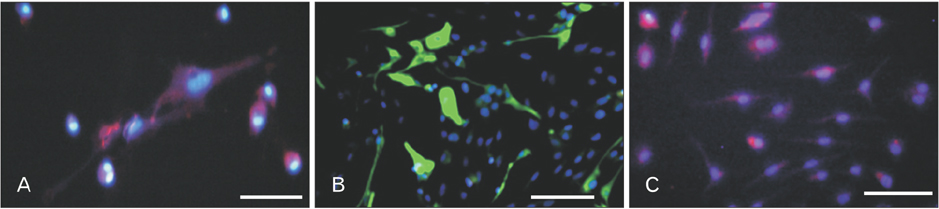
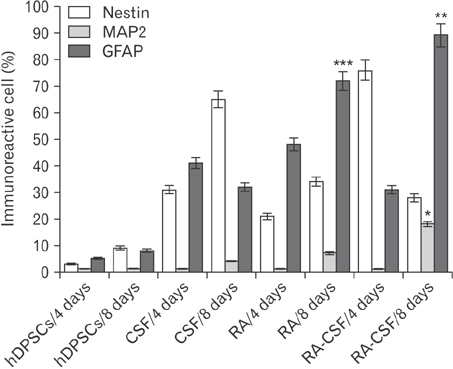
- Cerebrospinal fluid (CSF) contains several molecules which are essential for neurogenesis. Human dental pulp stem cells (hDPSCs) are putatively neural crest cell-derived that can differentiate into neurons and glial cells under appropriate neurotrophic factors. The aim of this study was to induce differentiation of hDPSCs into neuroglial phenotypes using retinoic acid (RA) and CSF. The hDPSCs from an impacted third molar were isolated by mechanical and digestion and cultured. The cells have treated by 10â»â·µM RA (RA group) for 8 days, 10% CSF (CSF group) for 8 days and RA with CSF for 8 days (RA/CSF group). Nestin, microtubule-associated protein 2 (MAP2), and glial fibrillary acidic protein immunostaining were used to examine the differentiated cells. Axonal outgrowth was detected using Bielschowsky's silver impregnation method and Nissl bodies were stained in differentiated cells by Cresyl violet. The morphology of differentiated cells in treated groups was significantly changed after 3-5 days. The results of immunocytochemistry showed the presence of neuroprogenitor marker nestin was seen in all groups. However, the high percentage of nestin positive cells and MAP2, as mature neural markers, were observed at the pre-induction and induction stage, respectively. Nissl bodies were detected as dark-blue particles in the cytoplasm of treated cells. Our findings showed the RA as pre-inducer and CSF as inducer for using in vitro differentiation of neuron-like cells and neuroglial cells from hDPSCs.
Keyword
MeSH Terms
-
Axons
Cerebrospinal Fluid*
Cytoplasm
Dental Pulp*
Digestion
Glial Fibrillary Acidic Protein
Humans*
Immunohistochemistry
In Vitro Techniques
Methods
Microtubule-Associated Proteins
Molar, Third
Nerve Growth Factors
Nestin
Neural Crest
Neurogenesis
Neuroglia*
Neurons
Nissl Bodies
Phenotype
Silver
Stem Cells*
Tretinoin*
Viola
Glial Fibrillary Acidic Protein
Microtubule-Associated Proteins
Nerve Growth Factors
Silver
Tretinoin
Figure
Cited by 2 articles
-
Role of cerebrospinal fluid in differentiation of human dental pulp stem cells into neuron-like cells
Ghazaleh Goudarzi, Hatef Ghasemi Hamidabadi, Maryam Nazm Bojnordi, Azim Hedayatpour, Ali Niapour, Maria Zahiri, Forouzan Absalan, Shahram Darabi
Anat Cell Biol. 2020;53(3):292-300. doi: 10.5115/acb.19.241.Role of agmatine in the application of neural progenitor cell in central nervous system diseases: therapeutic potentials and effects
Renée Kosonen, Sumit Barua, Jong Youl Kim, Jong Eun Lee
Anat Cell Biol. 2021;54(2):143-151. doi: 10.5115/acb.21.089.
Reference
-
1. Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008; 26:1787–1795.2. Nazm Bojnordi M, Movahedin M, Tiraihi T, Javan M. Alteration in genes expression patterns during in vitro differentiation of mouse spermatogonial cells into neuroepithelial-like cells. Cytotechnology. 2013; 65:97–104.3. Buddensiek J, Dressel A, Kowalski M, Runge U, Schroeder H, Hermann A, Kirsch M, Storch A, Sabolek M. Cerebrospinal fluid promotes survival and astroglial differentiation of adult human neural progenitor cells but inhibits proliferation and neuronal differentiation. BMC Neurosci. 2010; 11:48.4. Chang CC, Chang KC, Tsai SJ, Chang HH, Lin CP. Neurogenic differentiation of dental pulp stem cells to neuron-like cells in dopaminergic and motor neuronal inductive media. J Formos Med Assoc. 2014; 113:956–965.5. Darabi S, Tiraihi T, Delshad A, Sadeghizadeh M. A new multistep induction protocol for the transdifferentiation of bone marrow stromal stem cells into GABAergic neuron-like cells. Iran Biomed J. 2013; 17:8–14.6. Bojnordi MN, Azizi H, Skutella T, Movahedin M, Pourabdolhossein F, Shojaei A, Hamidabadi HG. Differentiation of spermatogonia stem cells into functional mature neurons characterized with differential gene expression. Mol Neurobiol. 2016; 09. 19. [Epub]. DOI: 10.1007/s12035-016-0097-7.7. Darabi S, Tiraihi T, Ruintan A, Abbaszadeh HA, Delshad A, Taheri T. Polarized neural stem cells derived from adult bone marrow stromal cells develop a rosette-like structure. In Vitro Cell Dev Biol Anim. 2013; 49:638–652.8. Engberg N, Kahn M, Petersen DR, Hansson M, Serup P. Retinoic acid synthesis promotes development of neural progenitors from mouse embryonic stem cells by suppressing endogenous, Wnt-dependent nodal signaling. Stem Cells. 2010; 28:1498–1509.9. Gato A, Moro JA, Alonso MI, Bueno D, De La Mano A, Martín C. Embryonic cerebrospinal fluid regulates neuroepithelial survival, proliferation, and neurogenesis in chick embryos. Anat Rec A Discov Mol Cell Evol Biol. 2005; 284:475–484.10. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000; 97:13625–13630.11. Huang AH, Snyder BR, Cheng PH, Chan AW. Putative dental pulp-derived stem/stromal cells promote proliferation and differentiation of endogenous neural cells in the hippocampus of mice. Stem Cells. 2008; 26:2654–2663.12. Karaöz E, Demircan PC, Sağlam O, Aksoy A, Kaymaz F, Duruksu G. Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochem Cell Biol. 2011; 136:455–473.13. Karaoz E, Dogan BN, Aksoy A, Gacar G, Akyüz S, Ayhan S, Genç ZS, Yürüker S, Duruksu G, Demircan PC, Sariboyaci AE. Isolation and in vitro characterisation of dental pulp stem cells from natal teeth. Histochem Cell Biol. 2010; 133:95–112.14. Laino G, d'Aquino R, Graziano A, Lanza V, Carinci F, Naro F, Pirozzi G, Papaccio G. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). J Bone Miner Res. 2005; 20:1394–1402.15. Lehtinen MK, Walsh CA. Neurogenesis at the brain-cerebrospinal fluid interface. Annu Rev Cell Dev Biol. 2011; 27:653–679.16. Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M, Maynard T, Gonzalez D, Kim S, Ye P, D'Ercole AJ, Wong ET, LaMantia AS, Walsh CA. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011; 69:893–905.17. Lei M, Li K, Li B, Gao LN, Chen FM, Jin Y. Mesenchymal stem cell characteristics of dental pulp and periodontal ligament stem cells after in vivo transplantation. Biomaterials. 2014; 35:6332–6343.18. Martens W, Bronckaers A, Politis C, Jacobs R, Lambrichts I. Dental stem cells and their promising role in neural regeneration: an update. Clin Oral Investig. 2013; 17:1969–1983.19. Mashayekhi F, Azari M, Moghadam LM, Yazdankhah M, Naji M, Salehi Z. Changes in cerebrospinal fluid nerve growth factor levels during chick embryonic development. J Clin Neurosci. 2009; 16:1334–1337.20. Nabiuni M, Rasouli J, Parivar K, Kochesfehani HM, Irian S, Miyan JA. In vitro effects of fetal rat cerebrospinal fluid on viability and neuronal differentiation of PC12 cells. Fluids Barriers CNS. 2012; 9:8.21. Nakashima M, Iohara K, Sugiyama M. Human dental pulp stem cells with highly angiogenic and neurogenic potential for possible use in pulp regeneration. Cytokine Growth Factor Rev. 2009; 20:435–440.22. Nazm Bojnordi M, Ghasemi HH, Akbari E. Remyelination after lysophosphatidyl choline-induced demyelination is stimulated by bone marrow stromal cell-derived oligoprogenitor cell transplantation. Cells Tissues Organs. 2015; 200:300–306.23. Tehranipour M, Baharara J, Mostafaee M. The neuroprotective effect of CSF intraperitoneal injection on alpha motor degeneration after sciatic nerve compression in rat. Arak Med Univ J. 2009; 12:101–108.24. Otify DY, Youssef EA, Nagy NB, Marei MK, Youssif MI. Transdifferentiation of bone marrow mesenchymal stem cells into neural cells via cerebrospinal fluid. Biomed Biotechnol. 2014; 2:66–79.25. Nazm Bojnordi M, Movahedin M, Tiraihi T, Javan M, Ghasemi Hamidabadi H. Oligoprogenitor cells derived from spermatogonia stem cells improve remyelination in demyelination model. Mol Biotechnol. 2014; 56:387–393.26. Ghasemi Hamidabadi H, Rezvani Z, Nazm Bojnordi M, Shirinzadeh H, Seifalian AM, Joghataei MT, Razaghpour M, Alibakhshi A, Yazdanpanah A, Salimi M, Mozafari M, Urbanska AM, Reis RL, Kundu SC, Gholipourmalekabadi M. Chitosan-intercalated montmorillonite/poly(vinyl alcohol) nanofibers as a platform to guide neuronlike differentiation of human dental pulp stem cells. ACS Appl Mater Interfaces. 2017; 9:11392–11404.27. Osathanon T, Nowwarote N, Pavasant P. Basic fibroblast growth factor inhibits mineralization but induces neuronal differentiation by human dental pulp stem cells through a FGFR and PLC-gamma signaling pathway. J Cell Biochem. 2011; 112:1807–1816.28. Salehi Z, Mashayekhi F, Naji M, Pandamooz S. Insulin-like growth factor-1 and insulin-like growth factor binding proteins in cerebrospinal fluid during the development of mouse embryos. J Clin Neurosci. 2009; 16:950–953.29. Yu J, He H, Tang C, Zhang G, Li Y, Wang R, Shi J, Jin Y. Differentiation potential of STRO-1+ dental pulp stem cells changes during cell passaging. BMC Cell Biol. 2010; 11:32.30. Zappaterra MW, Lehtinen MK. The cerebrospinal fluid: regulator of neurogenesis, behavior, and beyond. Cell Mol Life Sci. 2012; 69:2863–2878.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of cerebrospinal fluid in differentiation of human dental pulp stem cells into neuron-like cells
- Dental-derived cells for regenerative medicine: stem cells, cell reprogramming, and transdifferentiation
- Characterization of Human Dental Pulp Cells from Supernumerary Teeth by Using Flow Cytometry Analysis
- A study on differentiation potency of adult stem cells from pulp, periodontal ligament, and dental follicle to osteoblast
- Establishing Three-Dimensional Explant Culture of Human Dental Pulp Tissue