Int J Stem Cells.
2024 Aug;17(3):330-336. 10.15283/ijsc23105.
Establishing Three-Dimensional Explant Culture of Human Dental Pulp Tissue
- Affiliations
-
- 1Dental and Life Science Institute, School of Dentisty, Pusan National University, Yangsan, Korea
- 2Tissue Regeneration Laboratory, StemDen Co., Ltd., Yangsan, Korea
- 3Department of Pediatric Dentistry, School of Dentistry, Pusan National University, Yangsan, Korea
- 4Dental Research Institute, Pusan National University Dental Hospital, Yangsan, Korea
- 5Center for Electron Microscopy Research, Korea Basic Science Institute, Ochang, Korea
- 6Department of Physiology, The University of Tennessee Health Science Center, Memphis, TN, USA
- KMID: 2558610
- DOI: http://doi.org/10.15283/ijsc23105
Abstract
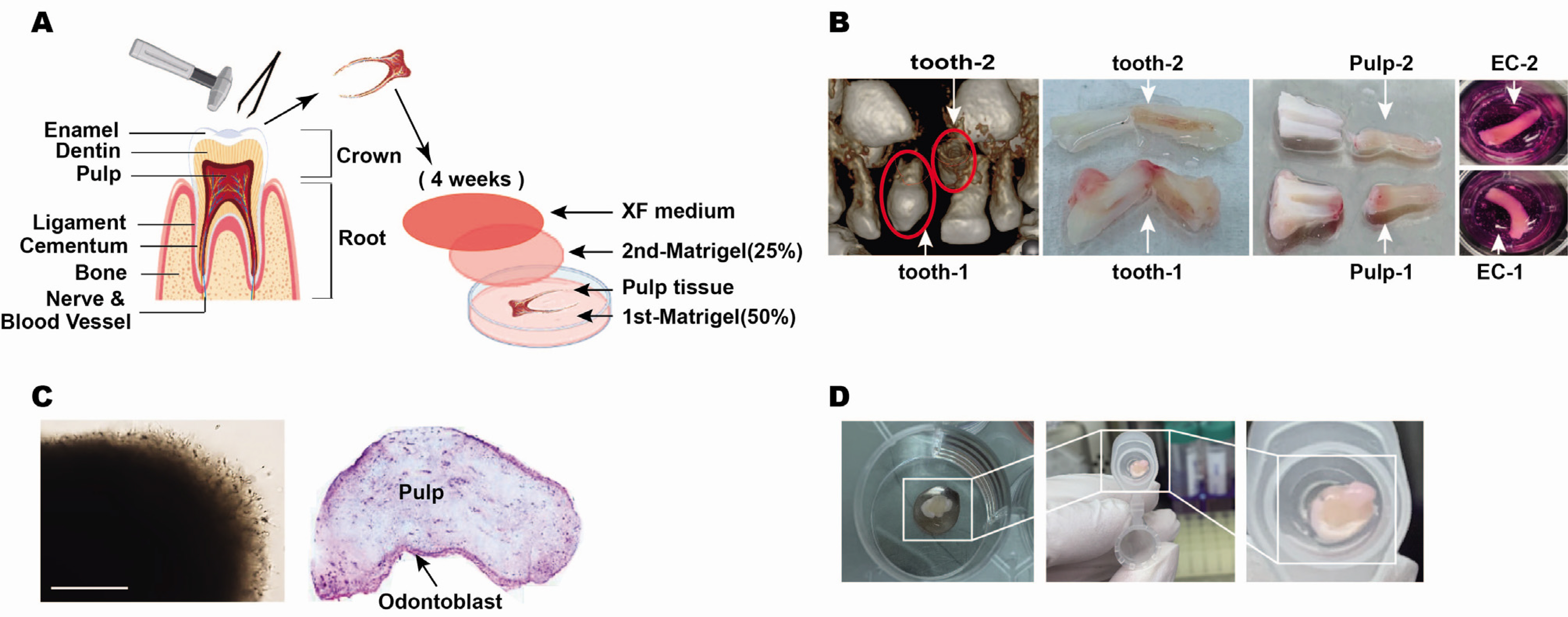
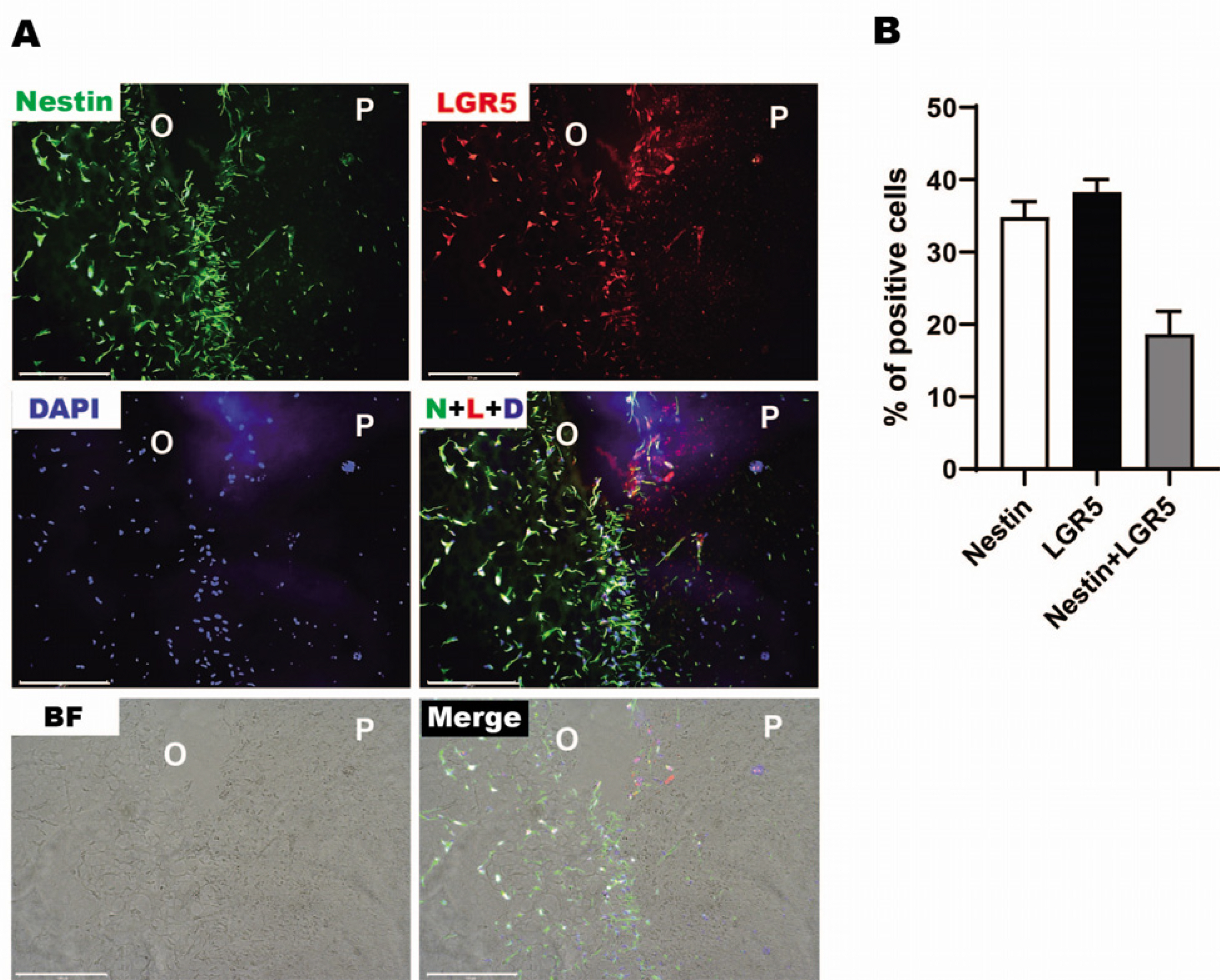
- Mesenchymal stem cells in the dental tissue indicate a disposition for differentiation into diverse dental lineages and contain enormous potential as the important means for regenerative medicine in dentistry. Among various dental tissues, the dental pulp contains stem cells, progenitor cells and odontoblasts for maintaining dentin homeostasis. The conventional culture of stem cells holds a limit as the living tissue constitutes the three-dimensional (3D) structure. Recent development in the organoid cultures have successfully recapitulated 3D structure and advanced to the assembling of different types. In the current study, the protocol for 3D explant culture of the human dental pulp tissue has been established by adopting the organoid culture. After isolating dental pulp from human tooth, the intact tissue was placed between two layers for Matrigel with addition of the culture medium. The reticular outgrowth of pre-odontoblast layer continued for a month and the random accumulation of dentin was observed near the end. Electron microscopy showed the cellular organization and in situ development of dentin, and immunohistochemistry exhibited the expression of odontoblast and stem cell markers in the outgrowth area. Three-dimensional explant culture of human dental pulp will provide a novel platform for understanding stem cell biology inside the tooth and developing the regenerative medicine.
Keyword
Figure
Reference
-
References
1. Arola DD, Gao S, Zhang H, Masri R. 2017; The tooth: its structure and properties. Dent Clin North Am. 61:651–668. DOI: 10.1016/j.cden.2017.05.001. PMID: 28886762. PMCID: PMC5774624.2. Gil-Bona A, Bidlack FB. 2020; Tooth enamel and its dynamic protein matrix. Int J Mol Sci. 21:4458. DOI: 10.3390/ijms21124458. PMID: 32585904. PMCID: PMC7352428.3. Chun K, Choi H, Lee J. 2014; Comparison of mechanical property and role between enamel and dentin in the human teeth. J Dent Biomech. 5:1758736014520809. DOI: 10.1177/1758736014520809. PMID: 24550998. PMCID: PMC3924884.4. Bae YC, Yoshida A. 2020; Morphological foundations of pain processing in dental pulp. J Oral Sci. 62:126–130. DOI: 10.2334/josnusd.19-0451. PMID: 32224566.5. Demarco FF, Conde MC, Cavalcanti BN, Casagrande L, Sakai VT, Nör JE. 2011; Dental pulp tissue engineering. Braz Dent J. 22:3–13. DOI: 10.1590/s0103-64402011000100001. PMID: 21519641. PMCID: PMC3736569.6. Yu C, Abbott PV. 2007; An overview of the dental pulp: its functions and responses to injury. Aust Dent J. 52(1 Suppl):S4–S16. DOI: 10.1111/j.1834-7819.2007.tb00525.x. PMID: 17546858.7. Guerrero-Jiménez M, Nic-Can GI, Castro-Linares N, et al. 2019; In vitro histomorphometric comparison of dental pulp tissue in different teeth. PeerJ. 7:e8212. DOI: 10.7287/peerj.8212v0.2/reviews/1. PMID: 31824782. PMCID: PMC6901003.8. Gopinath VK, Soumya S, Jayakumar MN. 2020; Osteogenic and odontogenic differentiation potential of dental pulp stem cells isolated from inflamed dental pulp tissues (I-DPSCs) by two different methods. Acta Odontol Scand. 78:281–289. DOI: 10.1080/00016357.2019.1702716. PMID: 31855089.9. Marrelli M, Codispoti B, Shelton RM, et al. 2018; Dental pulp stem cell mechanoresponsiveness: effects of mechanical stimuli on dental pulp stem cell behavior. Front Physiol. 9:1685. DOI: 10.3389/fphys.2018.01685. PMID: 30534086. PMCID: PMC6275199.10. Linde A. 1995; Dentin mineralization and the role of odontoblasts in calcium transport. Connect Tissue Res. 33:163–170. DOI: 10.3109/03008209509016997. PMID: 7554949.11. Couve E, Osorio R, Schmachtenberg O. 2013; The amazing odontoblast: activity, autophagy, and aging. J Dent Res. 92:765–772. DOI: 10.1177/0022034513509380. PMID: 23803461.12. McKee C, Chaudhry GR. 2017; Advances and challenges in stem cell culture. Colloids Surf B Biointerfaces. 159:62–77. DOI: 10.1016/j.colsurfb.2017.07.051. PMID: 28780462.13. Hsiao HY, Nien CY, Hong HH, Cheng MH, Yen TH. 2021; Application of dental stem cells in three-dimensional tissue regeneration. World J Stem Cells. 13:1610–1624. DOI: 10.4252/wjsc.v13.i11.1610. PMID: 34909114. PMCID: PMC8641025.14. Metzger W, Rother S, Pohlemann T, et al. 2017; Evaluation of cell-surface interaction using a 3D spheroid cell culture model on artificial extracellular matrices. Mater Sci Eng C Mater Biol Appl. 73:310–318. DOI: 10.1016/j.msec.2016.12.087. PMID: 28183614.15. Jorgenson AJ, Choi KM, Sicard D, et al. 2017; TAZ activation drives fibroblast spheroid growth, expression of profibrotic paracrine signals, and context-dependent ECM gene expression. Am J Physiol Cell Physiol. 312:C277–C285. DOI: 10.1152/ajpcell.00205.2016. PMID: 27881410. PMCID: PMC5401948.16. Chan YH, Lee YC, Hung CY, Yang PJ, Lai PC, Feng SW. 2021; Three-dimensional spheroid culture enhances multipotent differentiation and stemness capacities of human dental pulp-derived mesenchymal stem cells by modulating MAPK and NF-kB signaling pathways. Stem Cell Rev Rep. 17:1810–1826. DOI: 10.1007/s12015-021-10172-4. PMID: 33893620.17. DeVincenzo JP. 1968; An organ culture technique for maintaining the pulp tissue of intact human teeth. Exp Cell Res. 50:541–552. DOI: 10.1016/0014-4827(68)90417-5. PMID: 5663066.18. Sloan AJ, Shelton RM, Hann AC, Moxham BJ, Smith AJ. 1998; An in vitro approach for the study of dentinogenesis by organ culture of the dentine-pulp complex from rat incisor teeth. Arch Oral Biol. 43:421–430. DOI: 10.1016/s0003-9969(98)00029-6. PMID: 9717580.19. Zaw ZCT, Kawashima N, Kaneko T, Okiji T. 2022; Angiogenesis during coronal pulp regeneration using rat dental pulp cells: neovascularization in rat molars in vivo and proangiogenic dental pulp cell-endothelial cell interactions in vitro. J Dent Sci. 17:1160–1168. DOI: 10.1016/j.jds.2022.01.006. PMID: 35784152. PMCID: PMC9236944.20. Kapałczyńska M, Kolenda T, Przybyła W, et al. 2018; 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch Med Sci. 14:910–919. DOI: 10.5114/aoms.2016.63743. PMID: 30002710. PMCID: PMC6040128.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increased SOX2 expression in three-dimensional sphere culture of dental pulp stem cells
- Effect of hypoxia on angiogenesis-related proteins in human dental pulp cells
- Interleukin-8 and MCP(Monocyte Chemoattractant Protein)-1 expression by the Human Dental Pulps in cultures stimulated with Substance P
- Tissue viability of human placental explants in normoxic and hypoxic culture
- Expression of P2X3 and its colocalization with trpv1 in the human dental pulp