J Korean Acad Conserv Dent.
2005 May;30(3):193-203. 10.5395/JKACD.2005.30.3.193.
Interleukin-8 and MCP(Monocyte Chemoattractant Protein)-1 expression by the Human Dental Pulps in cultures stimulated with Substance P
- Affiliations
-
- 1Department of Conservative Dentistry, Division of Dentistry, Graduate School, Kyunghee University, Korea. gwchoi@khu.ac.kr
- 2Oral Biology Research Institute, College of Dentistry, Kyunghee University, Korea.
- KMID: 2045245
- DOI: http://doi.org/10.5395/JKACD.2005.30.3.193
Abstract
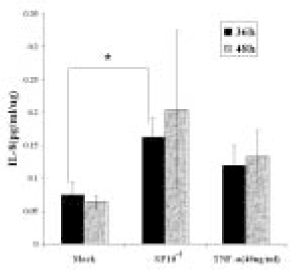
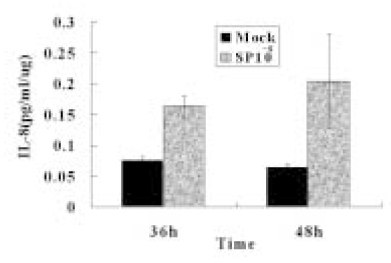
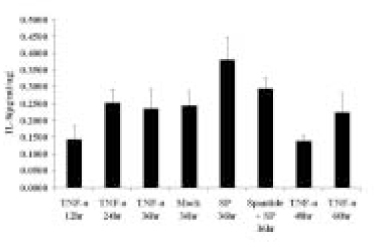
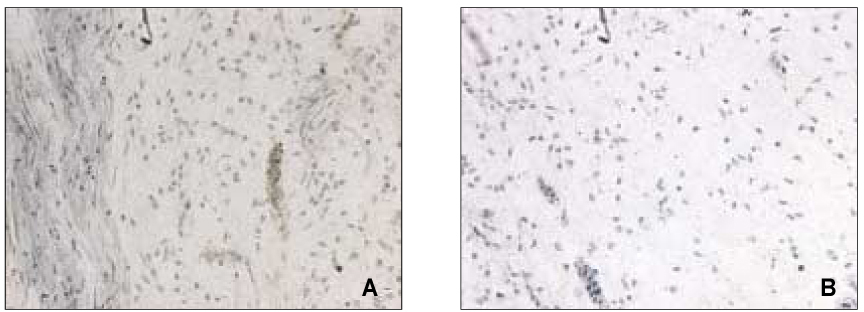
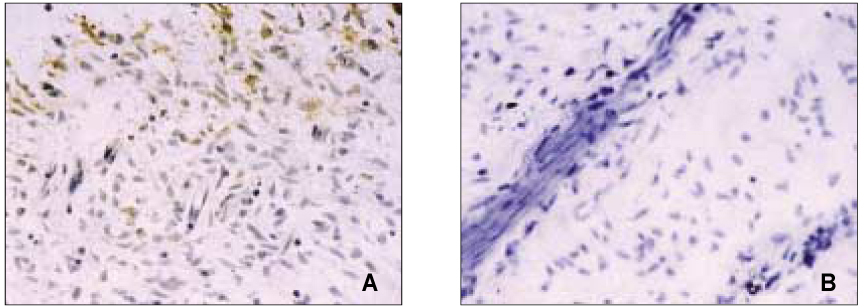
- The induction of the IL-8 and MCP-1 by the stimulation of Substance P and TNF-alpha (IL-8 agonist) and the specificity for SP using Spantide (SP antagonist) in the dental pulp tissues was measured quantitatively. In addition, the secretion of the IL-8 in the human dental pulp tissue 36 hrs after the stimulation of SP was observed after the stimulation of SP qualitatively. According to this study, the results were as follows: 1. There was the significant IL-8 induction at 36 h after SP (10-4M) stimulation of the pulp tissue comparing with the unstimulated dental pulp tissues (p < 0.05). IL-8 immunostaining was weakly detected along the periphery of the pulp tissue after Mock stimulation and IL-8 immunostaining was detected around the fibroblast in the pulp tissue 36h After SP (10-4M) stimulation, 2. The secretion of MCP-1 from the dental pulp tissues comparing with Mock stimulation was induced at 36 hrs after TNF-alpha (40 ng/ml) stimulation, but no induction with SP(10-4M). TNF-alpha (40 ng/ml) did not induce the IL-8 secretion from the pulp tissue, weak IL-8 immunostaining was detected along the periphery of the pulp tissue. 3. Spantide (10-5M) inhibited IL-8 induction from the pulp tissues 36 h after SP (10-4M) stimulation. These results suggest that SP significantly induces IL-8 recruiting neutrophils in localized human dental pulp tissue. MCP-1 appears to be less involved in the early establishment of pulpal inflammation in response to irritation such as mechanical insult of dentin. SP may have positive relation with the inflammation of the human dental pulp tissues.
Keyword
MeSH Terms
Figure
Reference
-
1. Stanley HR. Importance of the leukocyte to dental health. J Endod. 1977. 3:334–341.2. Bergenholtz G, Lindhe J. Effect of soluble plaque factors on inflammatory reactions in the dental pulp. Scand J Dent Res. 1975. 83:153–158.
Article3. Seltzer S, Bender IB, Ziontz M. The dynamics of pulp inflammation: correlations between diagnostic data and actual histologic findings in the pulp. Oral Surg Oral Med Oral Pathol. 1963. 16:846–871.
Article4. Graves DT, Jiang Y. Chemokines, a family of chemotactic cytokines. Crit Rev Oral Biol Med. 1995. 6:109–118.
Article5. Rauschenberger C, Bailey J, Cootauco C. Detection of human IL-2 in normal and inflamed dental pulps. J Endod. 1997. 23:366–370.
Article6. Nagaoka S, Yokuda M, Sakuta T, Taketoshi Y, Tamura M, Kakada H, Kawagoe M. Interleukin-8 gene expression by human dental pulp fibroblast in cultures stimulated with Prevotella intermedia Lipopolysaccharide. J Endod. 1996. 22:9–12.
Article7. Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989. 84:1045–1049.
Article8. Peveri P, Walz A, Dewald B, Baggiolini M. A novel neutrophil activating factor produced by human mononuclear phagocytes. J Exp Med. 1988. 167:1547–1559.
Article9. Jiang Y, Russel TR, Graves DT, Cheng H, Nong SH, Levitz SM. Monocyte chemoattractant protein 1 and Interleukin-8 production in mononuclear cells stimulated by oral microorganisms. Infect Immun. 1996. 64:4450–4455.
Article10. Yu X, Antoniades HN, Graves DT. Expression of monocyte chemoattractant protein 1 in human inflamed gingival tissues. Infect Immun. 1993. 61:4622–4628.
Article11. Tonetti MS, Imboden MA, Gerber L, Lang NP, Laissue J, Mueller C. Localized expression of mRNA for phagocyte-specific chemotactic cytokines in human periodontal infection. Infect Immun. 1994. 62:4005–4014.
Article12. Rahimi P, Wang CY, Stashenko P, Lee SK, Lorenzo JA, Graves DT. Monocyte Chemoattractant protein-1 expression and monocyte recruitment in osseous inflammation in the mouse. Endocrinology. 1995. 136:2752–2759.
Article13. Jontell M, Okiji T, Dahlgren U, Bergenholtz G. Immune defense mechanisms of the dental pulp. Crit Rev Oral Biol Med. 1998. 9:179–200.
Article14. Stashenko P, Teles R, D'Souza R. Periapical inflammatory responses and their modulation. Crit Rev Oral Biol Med. 1998. 9:498–521.
Article15. Byers MR, Narhi MV. Dental injury models; esperimental tools for understanding neuroinflammatory interactions and polymodal nociceptor functions. Crit Rev Oral Biol Med. 1999. 10:4–39.
Article16. Muller WA, Randolph GJ. Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J Leukoc Biol. 1999. 66:698–704.
Article17. Wakisaka S. Neuropeptides in the dental pulp: distribution, origins, and correlation. J Endod. 1990. 16:67–69.
Article18. Fristad I, Kvinnsland IH, Jonsson R, Heyeraas KJ. Effect of intermittent long-lasting electrical tooth stimulation on pulpal blood flow and immunocompetent cells: a hemodynamic and immunohistochemical study in young rat molars. Exp Neurol. 1997. 146:230–239.
Article19. Tran MT, Lausch RN, Oakes JE. Substance P differentially stimulates IL-8 synthesis in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2000. 41:3871–3877.20. Tran MT, Ritchie MH, Lausch RN, Oakes JE. Calcitonin gene-related peptide induces IL-8 synthesis in human corneal epithelial cells. J Immunol. 2000. 164:4307–4312.
Article21. Veronesi B, Carter JD, Devlin RB, Simon SA, Oortgiesen M. Neuropeptides and capsaicin stimulate the release of inlfammatory cytokines in a human broncheal epithelial cell line. Neuropeptides. 1999. 33:447–456.
Article22. Raap T, Justen HP, Miller LE, Cutolo M, Scholmerich J, Straub RH. Neurotransmitter modulation of interleukin 6 (IL-6) and IL-8 secretion of synovial fibroblasts in patients with rheumatoid arthritis compared to osteoarthritis. J Rheumatol. 2000. 27:2558–2565.23. Patel T, Park SH, Lin LM, Ciappelli F, Huang GT. Substance P induces interleukin-8 secretion from human dental pulp cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003. 96:478–485.
Article24. Hargreaves KM, Swift JQ, Roszkowski MT, Bowles W, Gary MG, Jackson DL. Pharmacology of peripheral neuropeptide and inflammatory mediator release. Oral Surg Oral Med Oral Pathol. 1994. 78:503–510.
Article25. Awawdeh L, Lundy FT, Shaw C, Lamey PJ, Linden GJ, Kennedy JG. Quantitative analysis of substance P, neurokinin A and calcitonin gene-related peptide in pulp tissue from painful and healthy human teeth. Int Endod J. 2002. 35:30–36.
Article26. Bowles WR, Withrow J, Lepinski A, Hargreaves KM. Tissue levels of immunoreactive substance P are increased in patients with irreversible pulpitis. J Endod. 2003. 29:265–267.
Article27. Foster CA, Mandak B, Kromer E, Rot A. Calcitonin gene-related peptide is chemotactic for human T lymphocytes. Ann N Y Acad Sci. 1992. 657:397–404.
Article28. Dunzendorfer S, Kaser A, Meierhofer C, Tilg H, Wiedermann CJ. Cutting edge: peripheral neuripeptides attract immature and arrest mature blood-derived dendritic cells. J Immunol. 2001. 166:2167–2172.
Article29. Roch-Arveiller M, Regoli D, Chanaud B, Lenoir M, Muntaner O, Stralzko S, Giroud JP. Tachykinins: effects on motility and metabolism of rat polymorphonuclear leucocytes. Pharmacology. 1986. 33:266–273.
Article30. Takahashi K. Changes in the pulpal vasculature during inflammation. J Endod. 1990. 16:92–97.
Article31. Fristad I, Vandevska-Radunovic V, Kvinnsland IH. Neurokinin-1 receptor expression in the mature dental pulp of rats. Arch Oral Biol. 1999. 44:191–195.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of substance P on the secretion of interleukin-8 and MCP (Monocyte Chemoattractant Protein)-1 from the Human Dental Pulp Cells
- Endothelin-1, Endothelin-2 and Endothelin-3 Induced Expression of Monocyte Chemoattractant Protein-1 in Rat Mesangial Cells
- Biophysically stressed vascular smooth muscle cells express MCP-1 via a PDGFR-ββ-HMGB1 signaling pathway
- Activated platelets induce secretion of interleukin-1beta, monocyte chemotactic protein-1, and macrophage inflammatory protein-1alpha and surface expression of intercellular adhesion molecule-1 on cultured endothelial cells
- Expression and Significance of Monocyte Chemoattractant Protein-1 in Inflammatory and Non-Inflammatory Glomerulonephritis