Korean J Physiol Pharmacol.
2024 Sep;28(5):449-456. 10.4196/kjpp.2024.28.5.449.
Biophysically stressed vascular smooth muscle cells express MCP-1 via a PDGFR-ββ-HMGB1 signaling pathway
- Affiliations
-
- 1Department of Pharmacology, School of Medicine, Pusan National University, Yangsan 50612, Korea
- 2Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan 50612, Korea
- KMID: 2559137
- DOI: http://doi.org/10.4196/kjpp.2024.28.5.449
Abstract
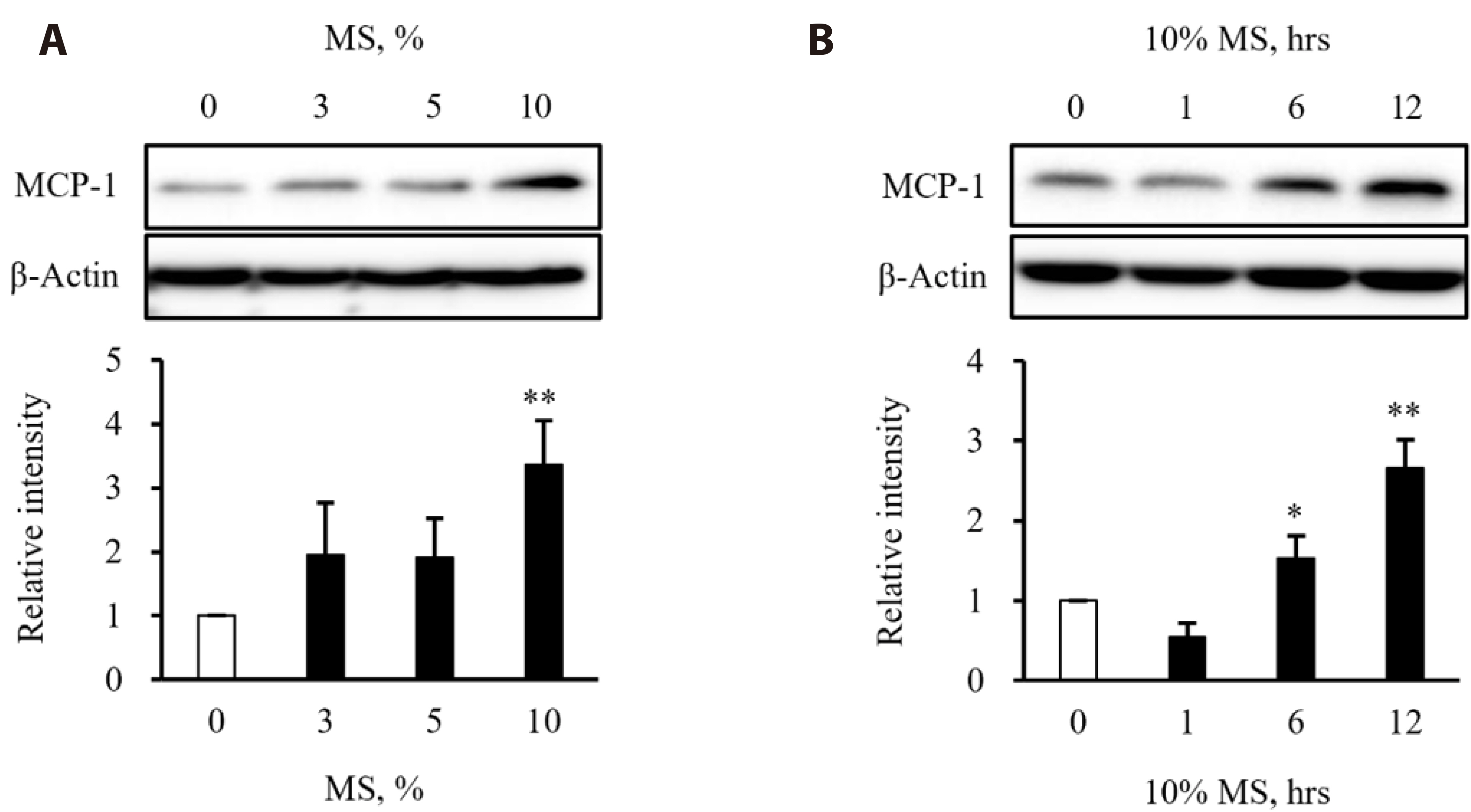
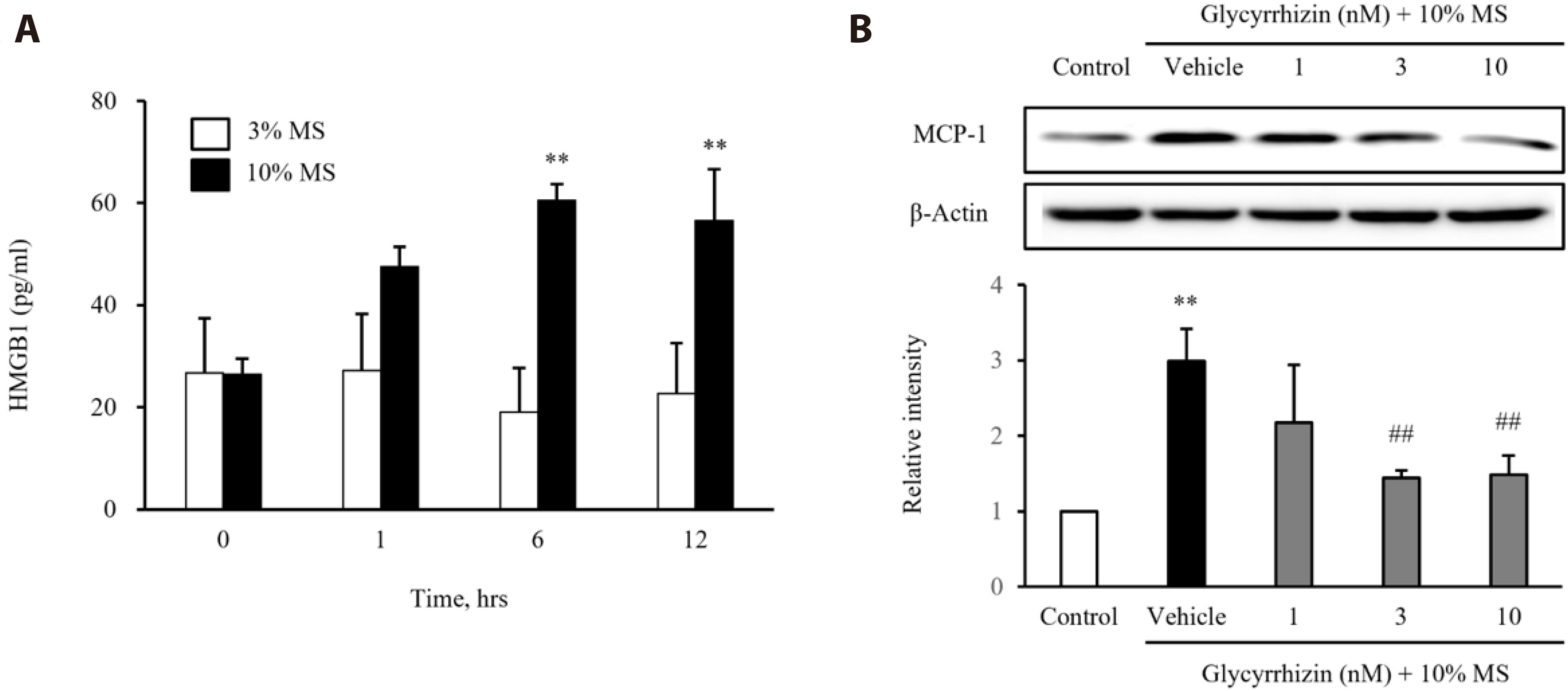
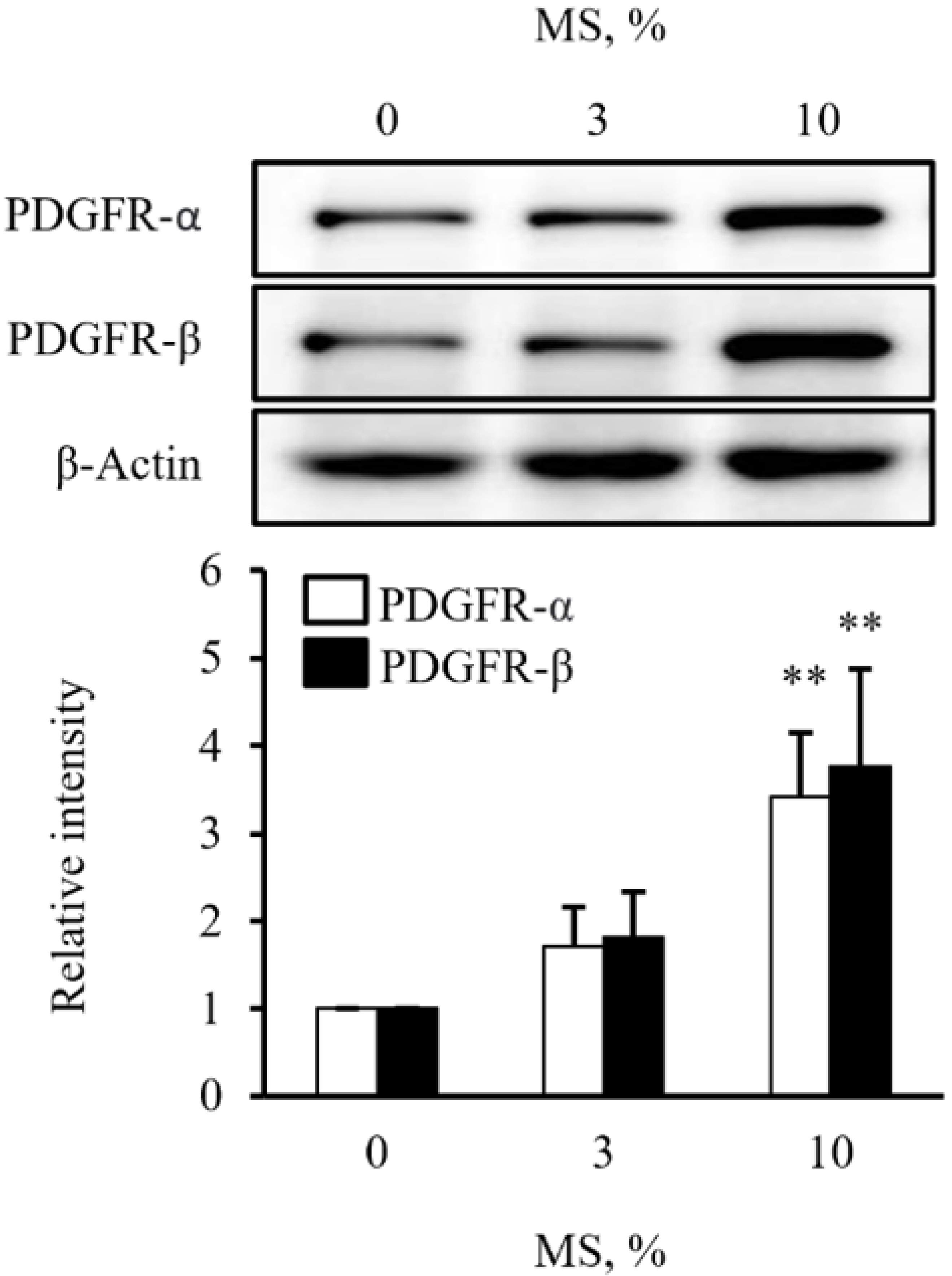
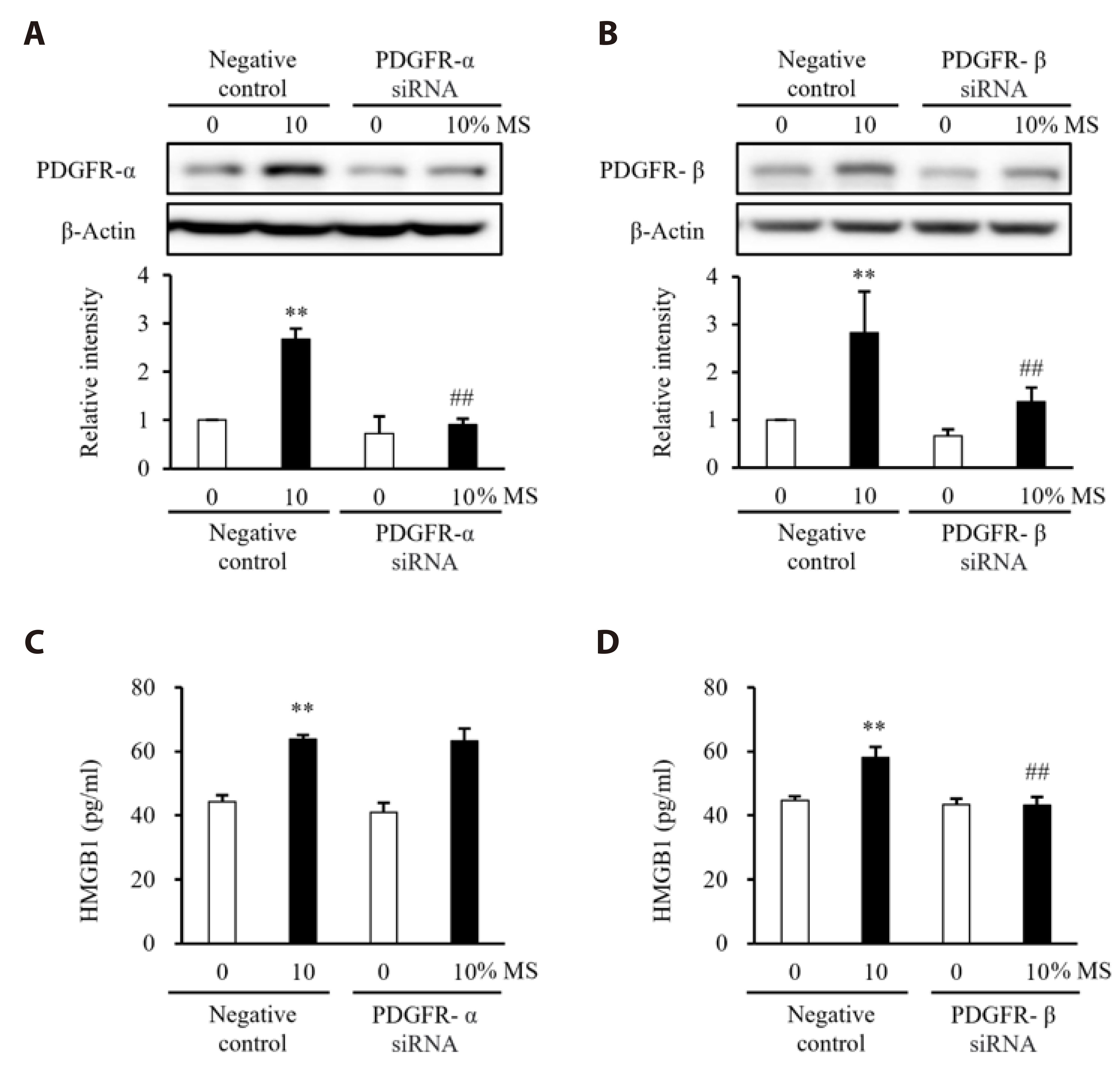
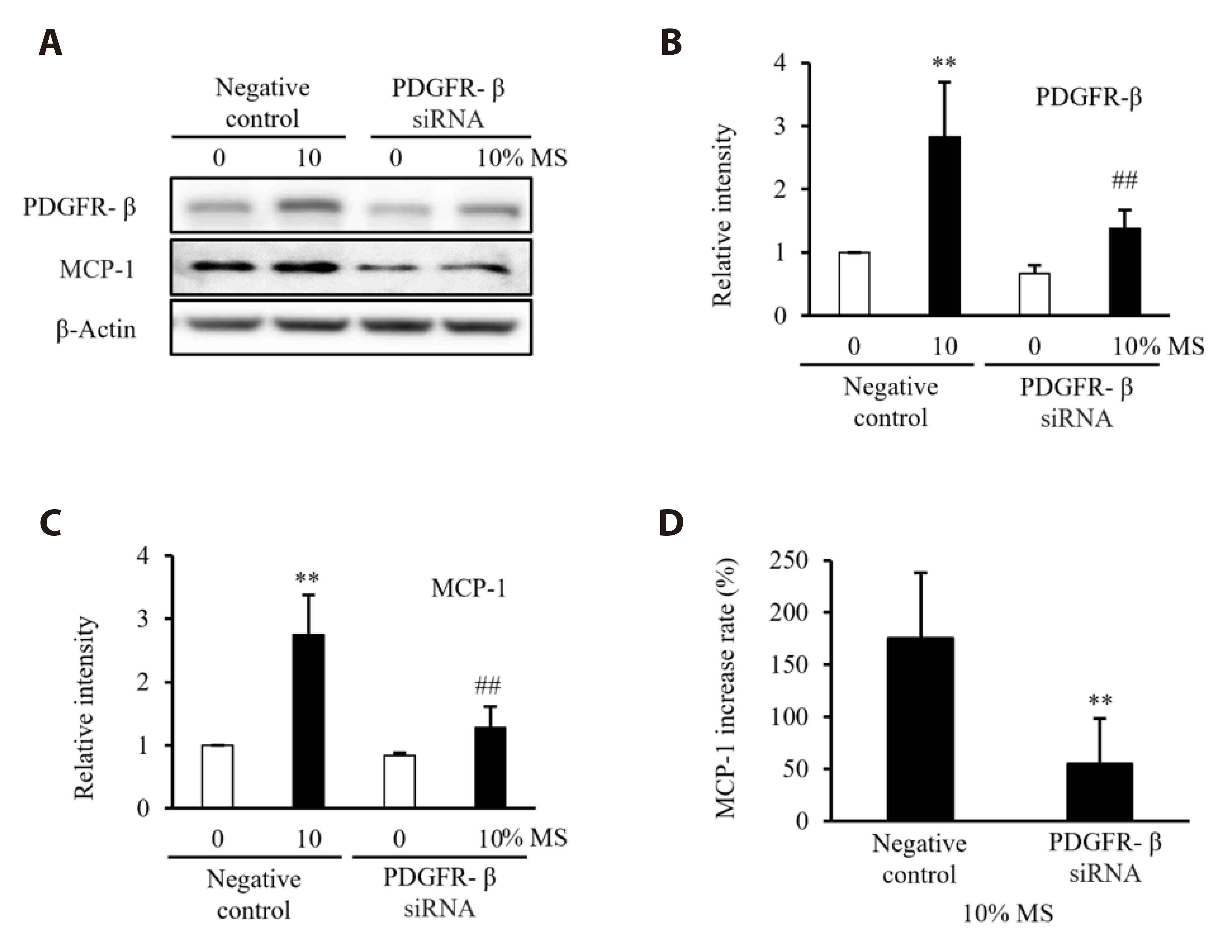
- Vascular smooth muscle cells (VSMCs) under biophysical stress play an active role in the progression of vascular inflammation, but the precise mechanisms are unclear. This study examined the cellular expression of monocyte chemoattractant protein 1 (MCP-1) and its related mechanisms using cultured rat aortic VSMCs stimulated with mechanical stretch (MS, equibiaxial cyclic stretch, 60 cycles/ min). When the cells were stimulated with 10% MS, MCP-1 expression was markedly increased compared to those in the cells stimulated with low MS intensity (3% or 5%). An enzyme-linked immunosorbent assay revealed an increase in HMGB1 released into culture media from the cells stimulated with 10% MS compared to those stimulated with 3% MS. A pretreatment with glycyrrhizin, a HMGB1 inhibitor, resulted in the marked attenuation of MCP-1 expression in the cells stimulated with 10% MS, suggesting a key role of HMGB1 on MCP-1 expression. Western blot analysis revealed higher PDGFR-α and PDGFR-β expression in the cells stimulated with 10% MS than 3% MS-stimulated cells. In the cells deficient of PDGFR-β using siRNA, but not PDGFR-α, HMGB1 released into culture media was significantly attenuated in the 10% MS-stimulated cells. Similarly, MCP-1 expression induced in 10% MS-stimulated cells was also attenuated in cells deficient of PDGFR-β. Overall, the PDGFR-β signaling plays a pivotal role in the increased expression of MCP-1 in VSMCs stressed with 10% MS. Therefore, targeting PDGFR-β signaling in VSMCs might be a promising therapeutic strategy for vascular complications in the vasculatures under excessive biophysical stress.
Keyword
Figure
Cited by 1 articles
-
Monotropein resists atherosclerosis by reducing inflammation, oxidative stress, and abnormal proliferation and migration of vascular smooth muscle cells
Hongliang Li, Bingqian Ye, Jiping Tian, Bofan Wang, Yiwen Zha, Shuying Zheng, Tan Ma, Wenwen Zhuang, Won Sun Park, Jingyan Liang
Korean J Physiol Pharmacol. 2025;29(2):245-255. doi: 10.4196/kjpp.24.352.
Reference
-
1. Wang D, Atanasov AG. 2019; The microRNAs regulating vascular smooth muscle cell proliferation: a minireview. Int J Mol Sci. 20:324. DOI: 10.3390/ijms20020324. PMID: 30646627. PMCID: PMC6359109.2. Jaminon A, Reesink K, Kroon A, Schurgers L. 2019; The role of vascular smooth muscle cells in arterial remodeling: focus on calcification-related processes. Int J Mol Sci. 20:5694. DOI: 10.3390/ijms20225694. PMID: 31739395. PMCID: PMC6888164.3. Lv P, Zhang F, Yin YJ, Wang YC, Gao M, Xie XL, Zhao LL, Dong LH, Lin YL, Shu YN, Zhang DD, Liu GX, Han M. 2016; SM22α inhibits lamellipodium formation and migration via Ras-Arp2/3 signaling in synthetic VSMCs. Am J Physiol Cell Physiol. 311:C758–C767. DOI: 10.1152/ajpcell.00033.2016. PMID: 27629412.4. Humphrey JD. 2021; Mechanisms of vascular remodeling in hypertension. Am J Hypertens. 34:432–441. DOI: 10.1093/ajh/hpaa195. PMID: 33245319. PMCID: PMC8140657.5. Yu X, Li Z. 2014; MicroRNAs regulate vascular smooth muscle cell functions in atherosclerosis (review). Int J Mol Med. 34:923–933. DOI: 10.3892/ijmm.2014.1853. PMID: 25197940.6. Evans BR, Yerly A, van der Vorst EPC, Baumgartner I, Bernhard SM, Schindewolf M, Döring Y. 2022; Inflammatory mediators in atherosclerotic vascular remodeling. Front Cardiovasc Med. 9:868934. DOI: 10.3389/fcvm.2022.868934. PMID: 35600479. PMCID: PMC9114307.7. Andrassy M, Volz HC, Maack B, Schuessler A, Gitsioudis G, Hofmann N, Laohachewin D, Wienbrandt AR, Kaya Z, Bierhaus A, Giannitsis E, Katus HA, Korosoglou G. 2012; HMGB1 is associated with atherosclerotic plaque composition and burden in patients with stable coronary artery disease. PLoS One. 7:e52081. Erratum in: PLoS One. 2014;9:e99246. DOI: 10.1371/journal.pone.0052081. PMID: 23284878. PMCID: PMC3524090.8. Cai J, Yuan H, Wang Q, Yang H, Al-Abed Y, Hua Z, Wang J, Chen D, Wu J, Lu B, Pribis JP, Jiang W, Yang K, Hackam DJ, Tracey KJ, Billiar TR, Chen AF. 2015; HMGB1-driven inflammation and intimal hyperplasia after arterial injury involves cell-specific actions mediated by TLR4. Arterioscler Thromb Vasc Biol. 35:2579–2593. DOI: 10.1161/ATVBAHA.115.305789. PMID: 26515416. PMCID: PMC4880018.9. Shi Y, Li S, Song Y, Liu P, Yang Z, Liu Y, Quan K, Yu G, Fan Z, Zhu W. 2019; Nrf-2 signaling inhibits intracranial aneurysm formation and progression by modulating vascular smooth muscle cell phenotype and function. J Neuroinflammation. 16:185. DOI: 10.1186/s12974-019-1568-3. PMID: 31585542. PMCID: PMC6778377.10. Ha JM, Yun SJ, Jin SY, Lee HS, Kim SJ, Shin HK, Bae SS. 2017; Regulation of vascular smooth muscle phenotype by cross-regulation of krüppel-like factors. Korean J Physiol Pharmacol. 21:37–44. DOI: 10.4196/kjpp.2017.21.1.37. PMID: 28066139. PMCID: PMC5214909.11. Lacolley P, Regnault V, Nicoletti A, Li Z, Michel JB. 2012; The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc Res. 95:194–204. DOI: 10.1093/cvr/cvs135. PMID: 22467316.12. Wang K, Li W, Yu Q, Guo B, Yang B, Zhang C, Li M, Li J, Hu S, Zheng Q, Song Z. 2017; High mobility group box 1 mediates interferon-γ-induced phenotypic modulation of vascular smooth muscle cells. J Cell Biochem. 118:518–529. DOI: 10.1002/jcb.25682. PMID: 27579780.13. Jang EJ, Kim H, Baek SE, Jeon EY, Kim JW, Kim JY, Kim CD. 2022; HMGB1 increases RAGE expression in vascular smooth muscle cells via ERK and p-38 MAPK-dependent pathways. Korean J Physiol Pharmacol. 26:389–396. DOI: 10.4196/kjpp.2022.26.5.389. PMID: 36039739. PMCID: PMC9437367.14. Zhang H, Yang K, Chen F, Liu Q, Ni J, Cao W, Hua Y, He F, Liu Z, Li L, Fan G. 2022; Role of the CCL2-CCR2 axis in cardiovascular disease: pathogenesis and clinical implications. Front Immunol. 13:975367. DOI: 10.3389/fimmu.2022.975367. PMID: 36110847. PMCID: PMC9470149.15. Smith SA, Newby AC, Bond M. 2019; Ending restenosis: inhibition of vascular smooth muscle cell proliferation by cAMP. Cells. 8:1447. DOI: 10.3390/cells8111447. PMID: 31744111. PMCID: PMC6912325.16. Ross R. 1995; Cell biology of atherosclerosis. Annu Rev Physiol. 57:791–804. DOI: 10.1146/annurev.ph.57.030195.004043. PMID: 7778883.17. Regan JK, Kannan PS, Kemp MW, Kramer BW, Newnham JP, Jobe AH, Kallapur SG. 2016; Damage-associated molecular pattern and fetal membrane vascular injury and collagen disorganization in lipopolysaccharide-induced intra-amniotic inflammation in fetal sheep. Reprod Sci. 23:69–80. DOI: 10.1177/1933719115594014. PMID: 26156854. PMCID: PMC5933192.18. Nishibori M, Mori S, Takahashi HK. 2019; Anti-HMGB1 monoclonal antibody therapy for a wide range of CNS and PNS diseases. J Pharmacol Sci. 140:94–101. DOI: 10.1016/j.jphs.2019.04.006. PMID: 31105025.19. Loperena R, Van Beusecum JP, Itani HA, Engel N, Laroumanie F, Xiao L, Elijovich F, Laffer CL, Gnecco JS, Noonan J, Maffia P, Jasiewicz-Honkisz B, Czesnikiewicz-Guzik M, Mikolajczyk T, Sliwa T, Dikalov S, Weyand CM, Guzik TJ, Harrison DG. 2018; Hypertension and increased endothelial mechanical stretch promote monocyte differentiation and activation: roles of STAT3, interleukin 6 and hydrogen peroxide. Cardiovasc Res. 114:1547–1563. DOI: 10.1093/cvr/cvy112. PMID: 29800237. PMCID: PMC6106108.20. Ghantous CM, Farhat R, Djouhri L, Alashmar S, Anlar G, Korashy HM, Agouni A, Zeidan A. 2020; Molecular mechanisms of adiponectin-induced attenuation of mechanical stretch-mediated vascular remodeling. Oxid Med Cell Longev. 2020:6425782. DOI: 10.1155/2020/6425782. PMID: 32566092. PMCID: PMC7260649.21. Wang A, Cao S, Stowe JC, Valdez-Jasso D. 2021; Substrate stiffness and stretch regulate profibrotic mechanosignaling in pulmonary arterial adventitial fibroblasts. Cells. 10:1000. DOI: 10.3390/cells10051000. PMID: 33922850. PMCID: PMC8146344.22. Land WG. 2013; Chronic allograft dysfunction: a model disorder of innate immunity. Biomed J. 36:209–228. DOI: 10.4103/2319-4170.117622. PMID: 24225188.23. Kalinina N, Agrotis A, Antropova Y, DiVitto G, Kanellakis P, Kostolias G, Ilyinskaya O, Tararak E, Bobik A. 2004; Increased expression of the DNA-binding cytokine HMGB1 in human atherosclerotic lesions: role of activated macrophages and cytokines. Arterioscler Thromb Vasc Biol. 24:2320–2325. DOI: 10.1161/01.ATV.0000145573.36113.8a. PMID: 15374849.24. Baek SE, Park SY, Bae SS, Kim K, Lee WS, Kim CD. 2018; BLTR1 in monocytes emerges as a therapeutic target for vascular inflammation with a subsequent intimal hyperplasia in a murine wire-injured femoral artery. Front Immunol. 9:1938. DOI: 10.3389/fimmu.2018.01938. PMID: 30210495. PMCID: PMC6121004.25. Zou JY, Crews FT. 2014; Release of neuronal HMGB1 by ethanol through decreased HDAC activity activates brain neuroimmune signaling. PLoS One. 9:e87915. DOI: 10.1371/journal.pone.0087915. PMID: 24551070. PMCID: PMC3925099.26. Jeon EY, Baek SE, Kim JO, Choi JM, Jang EJ, Kim CD. 2021; A pivotal role for AP-1-mediated osteopontin expression in the increased migration of vascular smooth muscle cells stimulated with HMGB1. Front Physiol. 12:775464. DOI: 10.3389/fphys.2021.775464. PMID: 34803747. PMCID: PMC8599980.27. Egashira K, Zhao Q, Kataoka C, Ohtani K, Usui M, Charo IF, Nishida K, Inoue S, Katoh M, Ichiki T, Takeshita A. 2002; Importance of monocyte chemoattractant protein-1 pathway in neointimal hyperplasia after periarterial injury in mice and monkeys. Circ Res. 90:1167–1172. DOI: 10.1161/01.RES.0000020561.03244.7E. PMID: 12065319.28. Deshmane SL, Kremlev S, Amini S, Sawaya BE. 2009; Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 29:313–326. DOI: 10.1089/jir.2008.0027. PMID: 19441883. PMCID: PMC2755091.29. Singh S, Anshita D, Ravichandiran V. 2021; MCP-1: function, regulation, and involvement in disease. Int Immunopharmacol. 101:107598. DOI: 10.1016/j.intimp.2021.107598. PMID: 34233864. PMCID: PMC8135227.30. Ylä-Herttuala S, Lipton BA, Rosenfeld ME, Särkioja T, Yoshimura T, Leonard EJ, Witztum JL, Steinberg D. 1991; Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci U S A. 88:5252–5256. DOI: 10.1073/pnas.88.12.5252. PMID: 2052604. PMCID: PMC51850.31. Viedt C, Vogel J, Athanasiou T, Shen W, Orth SR, Kübler W, Kreuzer J. 2002; Monocyte chemoattractant protein-1 induces proliferation and interleukin-6 production in human smooth muscle cells by differential activation of nuclear factor-kappaB and activator protein-1. Arterioscler Thromb Vasc Biol. 22:914–920. DOI: 10.1161/01.ATV.0000019009.73586.7F. PMID: 12067898.32. Kim J, Lee KP, Kim BS, Lee SJ, Moon BS, Baek S. 2020; Heat shock protein 90 inhibitor AUY922 attenuates platelet-derived growth factor-BB-induced migration and proliferation of vascular smooth muscle cells. Korean J Physiol Pharmacol. 24:241–248. DOI: 10.4196/kjpp.2020.24.3.241. PMID: 32392915. PMCID: PMC7193915.33. Park HS, Han JH, Jung SH, Lee DH, Heo KS, Myung CS. 2018; Anti-apoptotic effects of autophagy via ROS regulation in microtubule-targeted and PDGF-stimulated vascular smooth muscle cells. Korean J Physiol Pharmacol. 22:349–360. DOI: 10.4196/kjpp.2018.22.3.349. PMID: 29719457. PMCID: PMC5928348.34. Sudano I, Roas S, Noll G. 2011; Vascular abnormalities in essential hypertension. Curr Pharm Des. 17:3039–3044. DOI: 10.2174/138161211798157766. PMID: 21861833.35. Perros F, Montani D, Dorfmüller P, Durand-Gasselin I, Tcherakian C, Le Pavec J, Mazmanian M, Fadel E, Mussot S, Mercier O, Hervé P, Emilie D, Eddahibi S, Simonneau G, Souza R, Humbert M. 2008; Platelet-derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 178:81–88. DOI: 10.1164/rccm.200707-1037OC. PMID: 18420966.36. Kim JO, Baek SE, Jeon EY, Choi JM, Jang EJ, Kim CD. 2022; PDGFR-β signaling mediates HMGB1 release in mechanically stressed vascular smooth muscle cells. PLoS One. 17:e0265191. DOI: 10.1371/journal.pone.0265191. PMID: 35294955. PMCID: PMC8926240.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- HMGB1 increases RAGE expression in vascular smooth muscle cells via ERK and p-38 MAPK-dependent pathways
- Roles of PDGF/PDGFR signaling in various organs
- Echinochrome A inhibits HMGB1-induced vascular smooth muscle cell migration by suppressing osteopontin expression
- Effects of Monocyte Chemoattractant Protein-1 on Growth and Migration of Cultured Human Vascular Smooth Muscle Cells
- Ascochlorin Derivative, AS-6, Inhibits TNF-alpha-Induced fractalkine, MCP-1 and VCAM-1 Expression in Rat Aortic Smooth Muscle Cells