Korean J Physiol Pharmacol.
2019 Mar;23(2):113-120. 10.4196/kjpp.2019.23.2.113.
Mannosylerythritol lipids ameliorate ultraviolet A-induced aquaporin-3 downregulation by suppressing c-Jun N-terminal kinase phosphorylation in cultured human keratinocytes
- Affiliations
-
- 1R&D Center, Amorepacific Corporation, Yongin 17074, Korea.
- 2Department of Beauty and Cosmetic Science, Eulji University, Seongnam 13135, Korea. cslee2010@eulji.ac.kr
- 3Department of Veterinary Pathology, College of Veterinary Medicine, Seoul National University, Seoul 08826, Korea. daeyong@snu.ac.kr
- KMID: 2438085
- DOI: http://doi.org/10.4196/kjpp.2019.23.2.113
Abstract
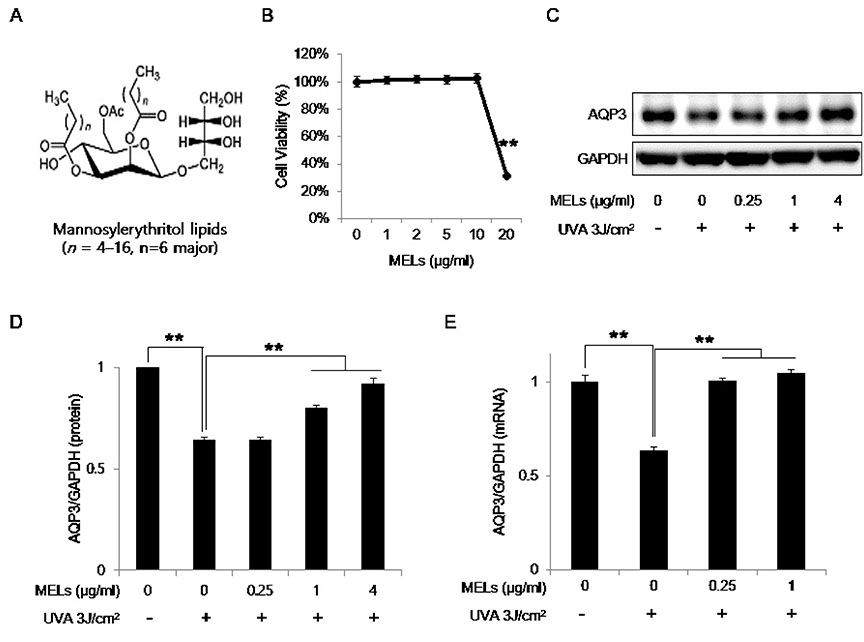
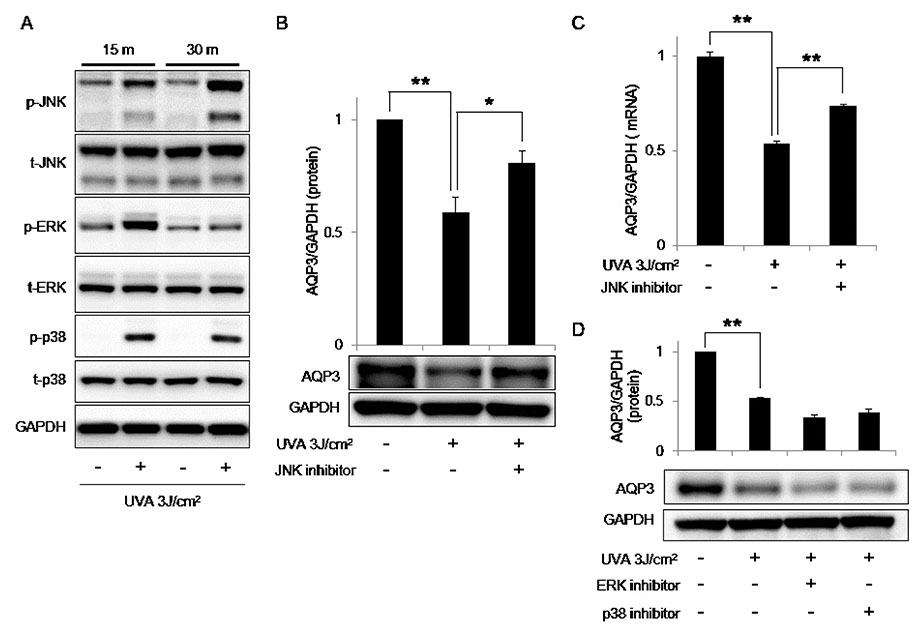
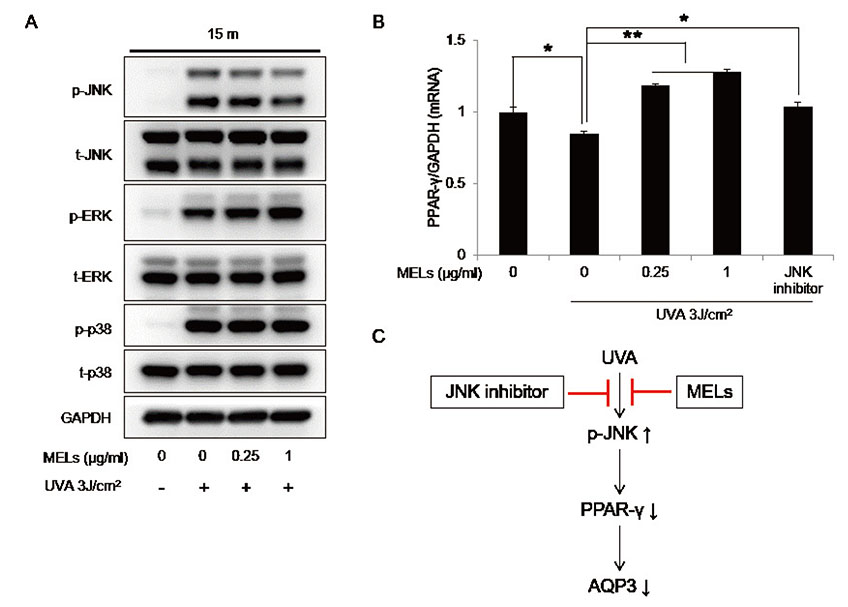
- Mannosylerythritol lipids (MELs) are glycolipids and have several pharmacological efficacies. MELs also show skin-moisturizing efficacy through a yet-unknown underlying mechanism. Aquaporin-3 (AQP3) is a membrane protein that contributes to the water homeostasis of the epidermis, and decreased AQP3 expression following ultraviolet (UV)-irradiation of the skin is associated with reduced skin moisture. No previous study has examined whether the skin-moisturizing effect of MELs might act through the modulation of AQP3 expression. Here, we report for the first time that MELs ameliorate the UVA-induced downregulation of AQP3 in cultured human epidermal keratinocytes (HaCaT keratinocytes). Our results revealed that UVA irradiation decreases AQP3 expression at the protein and messenger RNA (mRNA) levels, but that MEL treatment significantly ameliorated these effects. Our mitogen-activated protein kinase inhibitor analysis revealed that phosphorylation of c-Jun N-terminal kinase (JNK), but not extracellular signal-regulated kinase or p38, mediates UVA-induced AQP3 downregulation, and that MEL treatment significantly suppressed the UVA-induced phosphorylation of JNK. To explore a possible mechanism, we tested whether MELs could regulate the expression of peroxidase proliferator-activated receptor gamma (PPAR-γ), which acts as a potent transcription factor for AQP3 expression. Interestingly, UVA irradiation significantly inhibited the mRNA expression of PPAR-γ in HaCaT keratinocytes, whereas a JNK inhibitor and MELs significantly rescued this effect. Taken together, these findings suggest that MELs ameliorate UVA-induced AQP3 downregulation in HaCaT keratinocytes by suppressing JNK activation to block the decrease of PPAR-γ. Collectively, our findings suggest that MELs can be used as a potential ingredient that modulates AQP3 expression to improve skin moisturization following UVA irradiation-induced damage.
Keyword
MeSH Terms
-
Down-Regulation*
Epidermis
Glycolipids
Homeostasis
Humans*
JNK Mitogen-Activated Protein Kinases*
Keratinocytes*
Membrane Proteins
Peroxidase
Phosphorylation*
Phosphotransferases
PPAR gamma
Protein Kinases
RNA, Messenger
Skin
Transcription Factors
Water
Glycolipids
JNK Mitogen-Activated Protein Kinases
Membrane Proteins
PPAR gamma
Peroxidase
Phosphotransferases
Protein Kinases
RNA, Messenger
Transcription Factors
Water
Figure
Reference
-
1. Morita T, Fukuoka T, Imura T, Kitamoto D. Mannosylerythritol lipids: production and applications. J Oleo Sci. 2015; 64:133–141.
Article2. Kitamoto D, Yanagishita H, Shinbo T, Nakane T, Kamisawa C, Nakahara T. Surface active properties and antimicrobial activities of mannosylerythritol lipids as biosurfactants produced by Candida antarctica. J Biotechnol. 1993; 29:91–96.
Article3. Morita Y, Tadokoro S, Sasai M, Kitamoto D, Hirashima N. Biosurfactant mannosyl-erythritol lipid inhibits secretion of inflammatory mediators from RBL-2H3 cells. Biochim Biophys Acta. 2011; 1810:1302–1308.
Article4. Zhao X, Wakamatsu Y, Shibahara M, Nomura N, Geltinger C, Nakahara T, Murata T, Yokoyama KK. Mannosylerythritol lipid is a potent inducer of apoptosis and differentiation of mouse melanoma cells in culture. Cancer Res. 1999; 59:482–486.5. Wakamatsu Y, Zhao X, Jin C, Day N, Shibahara M, Nomura N, Nakahara T, Murata T, Yokoyama KK. Mannosylerythritol lipid induces characteristics of neuronal differentiation in PC12 cells through an ERK-related signal cascade. Eur J Biochem. 2001; 268:374–383.
Article6. Im JH, Yanagishita H, Ikegami T, Takeyama Y, Idemoto Y, Koura N, Kitamoto D. Mannosylerythritol lipids, yeast glycolipid biosurfactants, are potential affinity ligand materials for human immunoglobulin G. J Biomed Mater Res A. 2003; 65:379–385.
Article7. Ueno Y, Inoh Y, Furuno T, Hirashima N, Kitamoto D, Nakanishi M. NBD-conjugated biosurfactant (MEL-A) shows a new pathway for transfection. J Control Release. 2007; 123:247–253.
Article8. Morita T, Fukuoka T, Imura T, Kitamoto D. Production of mannosylerythritol lipids and their application in cosmetics. Appl Microbiol Biotechnol. 2013; 97:4691–4700.
Article9. Morita T, Kitagawa M, Suzuki M, Yamamoto S, Sogabe A, Yanagidani S, Imura T, Fukuoka T, Kitamoto D. A yeast glycolipid biosurfactant, mannosylerythritol lipid, shows potential moisturizing activity toward cultured human skin cells: the recovery effect of MELA on the SDS-damaged human skin cells. J Oleo Sci. 2009; 58:639–642.
Article10. Yamamoto S, Morita T, Fukuoka T, Imura T, Yanagidani S, Sogabe A, Kitamoto D, Kitagawa M. The moisturizing effects of glycolipid biosurfactants, mannosylerythritol lipids, on human skin. J Oleo Sci. 2012; 61:407–412.
Article11. Sugiyama Y, Ota Y, Hara M, Inoue S. Osmotic stress up-regulates aquaporin-3 gene expression in cultured human keratinocytes. Biochim Biophys Acta. 2001; 1522:82–88.
Article12. Hara-Chikuma M, Verkman AS. Aquaporin-3 functions as a glycerol transporter in mammalian skin. Biol Cell. 2005; 97:479–486.
Article13. Ma T, Hara M, Sougrat R, Verbavatz JM, Verkman AS. Impaired stratum corneum hydration in mice lacking epidermal water channel aquaporin-3. J Biol Chem. 2002; 277:17147–17153.
Article14. Hara M, Ma T, Verkman AS. Selectively reduced glycerol in skin of aquaporin-3-deficient mice may account for impaired skin hydration, elasticity, and barrier recovery. J Biol Chem. 2002; 277:46616–46621.
Article15. Li J, Tang H, Hu X, Chen M, Xie H. Aquaporin-3 gene and protein expression in sun-protected human skin decreases with skin ageing. Australas J Dermatol. 2010; 51:106–112.
Article16. Cao C, Wan S, Jiang Q, Amaral A, Lu S, Hu G, Bi Z, Kouttab N, Chu W, Wan Y. All-trans retinoic acid attenuates ultraviolet radiation-induced down-regulation of aquaporin-3 and water permeability in human keratinocytes. J Cell Physiol. 2008; 215:506–516.
Article17. Jeon BK, Kang MK, Lee GT, Lee KK, Lee HS, Woo WH, Mun YJ. EPA attenuates ultraviolet radiation-induced downregulation of aquaporin-3 in human keratinocytes. Arch Pharm Res. 2015; 38:1552–1560.
Article18. Ji C, Yang Y, Yang B, Xia J, Sun W, Su Z, Yu L, Shan S, He S, Cheng L, Wan Y, Bi Z. Trans-Zeatin attenuates ultraviolet induced down-regulation of aquaporin-3 in cultured human skin keratinocytes. Int J Mol Med. 2010; 26:257–263.
Article19. Shan SJ, Xiao T, Chen J, Geng SL, Li CP, Xu X, Hong Y, Ji C, Guo Y, Wei H, Liu W, Li D, Chen HD. Kanglaite attenuates UVB-induced down-regulation of aquaporin-3 in cultured human skin keratinocytes. Int J Mol Med. 2012; 29:625–629.
Article20. Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002; 12:9–18.
Article21. Cao C, Sun Y, Healey S, Bi Z, Hu G, Wan S, Kouttab N, Chu W, Wan Y. EGFR-mediated expression of aquaporin-3 is involved in human skin fibroblast migration. Biochem J. 2006; 400:225–234.
Article22. Camp HS, Tafuri SR, Leff T. c-Jun N-terminal kinase phosphorylates peroxisome proliferator-activated receptor-gamma1 and negatively regulates its transcriptional activity. Endocrinology. 1999; 140:392–397.23. Lee J, Lee J, Jung E, Kim YS, Roh K, Jung KH, Park D. Ultraviolet A regulates adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells via up-regulation of Kruppel-like factor 2. J Biol Chem. 2010; 285:32647–32656.
Article24. Tardelli M, Bruschi FV, Claudel T, Moreno-Viedma V, Halilbasic E, Marra F, Herac M, Stulnig TM, Trauner M. AQP3 is regulated by PPARXMLLink_XYZ and JNK in hepatic stellate cells carrying PNPLA3 I148M. Sci Rep. 2017; 7:14661.
Article25. Silvers AL, Bachelor MA, Bowden GT. The role of JNK and p38 MAPK activities in UVA-induced signaling pathways leading to AP-1 activation and c-Fos expression. Neoplasia. 2003; 5:319–329.
Article26. Hwang HS, Shim JH. Brazilin and Caesalpinia sappan L. extract protect epidermal keratinocytes from oxidative stress by inducing the expression of GPX7. Chin J Nat Med. 2018; 16:203–209.
Article27. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001; 25:402–408.28. Hara-Chikuma M, Verkman AS. Roles of aquaporin-3 in the epidermis. J Invest Dermatol. 2008; 128:2145–2151.
Article29. Kligman LH. The hairless mouse and photoaging. Photochem Photobiol. 1991; 54:1109–1118.
Article30. Morita T, Kitagawa M, Yamamoto S, Sogabe A, Imura T, Fukuoka T, Kitamoto D. Glycolipid biosurfactants, mannosylerythritol lipids, repair the damaged hair. J Oleo Sci. 2010; 59:267–272.
Article31. Takahashi M, Morita T, Fukuoka T, Imura T, Kitamoto D. Glycolipid biosurfactants, mannosylerythritol lipids, show antioxidant and protective effects against H2O2-induced oxidative stress in cultured human skin fibroblasts. J Oleo Sci. 2012; 61:457–464.32. Xie H, Liu F, Liu L, Dan J, Luo Y, Yi Y, Chen X, Li J. Protective role of AQP3 in UVA-induced NHSFs apoptosis via Bcl2 up-regulation. Arch Dermatol Res. 2013; 305:397–406.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Aquaporin-3 Downregulation in Vitiligo Keratinocytes Increases Oxidative Stress of Melanocytes
- Phloroglucinol Attenuates Ultraviolet B-Induced 8-Oxoguanine Formation in Human HaCaT Keratinocytes through Akt and ErkMediated Nrf2/Ogg1 Signaling Pathways
- The Effect of Supernatant from UVB - Irradiated Cultured Keratinocytes on the Growth , Melanin Content , and Tyrosinase Activity of Human Melanocyte
- Galangin (3,5,7-Trihydroxyflavone) Shields Human Keratinocytes from Ultraviolet B-Induced Oxidative Stress
- Inhibitory Action of 1,3,5-Trihydroxybenzene on UVB-Induced NADPH Oxidase 4 through AMPK and JNK Signaling Pathways