J Korean Ophthalmol Soc.
2019 Jan;60(1):55-61. 10.3341/jkos.2019.60.1.55.
Effect of Chronic Benzalkonium Chloride Exposure on Senescence in Trabecular Meshwork Cells
- Affiliations
-
- 1Cheil Eye Research Institute, Daegu, Korea. jck50ey@daum.net
- 2Cheil Eye Hospital, Daegu, Korea.
- KMID: 2431840
- DOI: http://doi.org/10.3341/jkos.2019.60.1.55
Abstract
- PURPOSE
To determine the possible effects of chronic exposure of low dose benzalkonium chloride (BAK) on trabecular meshwork cells, and to characterize the pathways involved in the effects.
METHODS
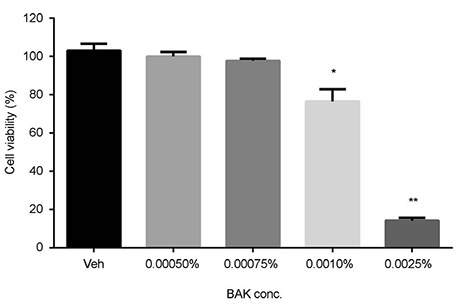
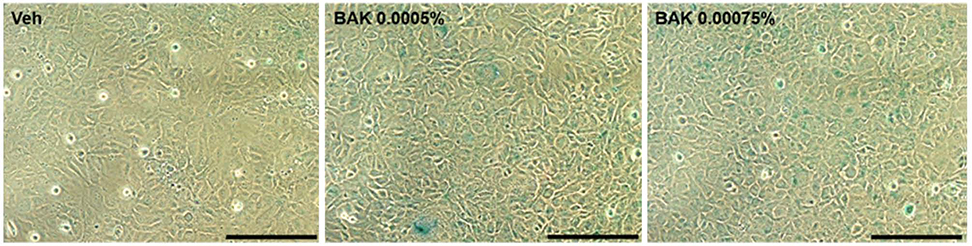
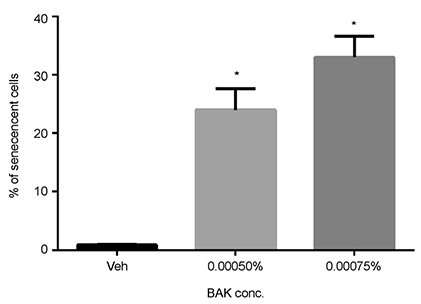
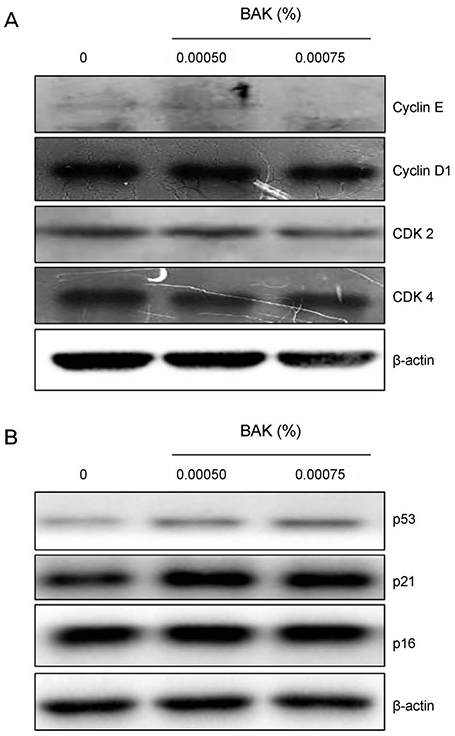
Trabecular meshwork cells were treated with 0.0005%, 0.00075%, 0.001%, and 0.0025% BAK for 10 minutes; then, the cells were transferred to a new medium for 24 hours. This process was repeated three times. Cell survival was assessed using the MTT assay to determine the non-apoptotic BAK concentration. Senescence-associated (SA)-β-gal staining was performed to compare quantitatively the cellular senescence of BAK-treated cells with the control group. Cells treated with BAK were analyzed by western blot to determine whether the expressions of cell cycle regulators were affected.
RESULTS
Two concentrations (0.0005% and 0.00075%) showed persistent cell viability and were chosen for further experiments. After SA-β-gal staining, cells treated with 0.0005% and 0.00075% BAK showed 28% (± 2.08), 37% (± 2.08) increases in cellular senescence expression, respectively, when compared with control cells (p < 0.05). To identify the molecular pathways involved in cell cycle arrest via BAK, western blot analysis was performed on trabecular meshwork cells, resulting in decreased expressions of cyclin E/CDK2, and increased expressions of the upper stream control molecules, p53 and p21.
CONCLUSIONS
Chronic exposure to low dose BAK accelerated cell senescence through cell cycle arrest. Because senescent cells of the trabecular meshwork can inhibit its outflow pathway function and ultimately worsen the glaucomatous process, long-term usage of topical glaucoma medications containing BAK should be conducted with caution.
MeSH Terms
Figure
Reference
-
1. Rasmussen CA, Kaufman PL, Kiland JA. Benzalkonium chloride and glaucoma. J Ocul Pharmacol Ther. 2014; 30:163–169.
Article2. Abu-Hassan DW, Acott TS, Kelley MJ. The trabecular meshwork: a basic review of form and function. J Ocul Biol. 2014; 2:https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4209746/. Accessed June 20, 2018.
Article3. Baudouin C, Denoyer A, Desbenoit N, et al. In vitro and in vivo experimental studies on trabecular meshwork degeneration induced by benzalkonium chloride (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2012; 110:40–63.4. Chang C, Zhang AQ, Kagan DB, et al. Mechanisms of benzalkonium chloride toxicity in a human trabecular meshwork cell line and the protective role of preservative-free tafluprost. Clin Exp Ophthalmol. 2015; 43:164–172.
Article5. Yu AL, Fuchshofer R, Kampik A, Welge-Lussen U. Effects of oxidative stress in trabecular meshwork cells are reduced by prostaglandin analogues. Invest Ophthalmol Vis Sci. 2008; 49:4872–4880.
Article6. Fechtner RD, Godfrey DG, Budenz D, et al. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010; 29:618–621.
Article7. Katz G, Springs CL, Craven ER, Montecchi-Palmer M. Ocular surface disease in patients with glaucoma or ocular hypertension treated with either BAK-preserved latanoprost or BAK-free travoprost. Clin Ophthalmol. 2010; 4:1253–1261.
Article8. Rossi GC, Pasinetti GM, Scudeller L, et al. Risk factors to develop ocular surface disease in treated glaucoma or ocular hypertension patients. Eur J Ophthalmol. 2013; 23:296–302.
Article9. Brignole-Baudouin F, Desbenoit N, Hamm G, et al. A new safety concern for glaucoma treatment demonstrated by mass spectrometry imaging of benzalkonium chloride distribution in the eye, an experimental study in rabbits. PLoS One. 2012; 7:e50180.
Article10. Desbenoit N, Schmitz-Afonso I, Baudouin C, et al. Localisation and quantification of benzalkonium chloride in eye tissue by TOF-SIMS imaging and liquid chromatography mass spectrometry. Anal Bioanal Chem. 2013; 405:4039–4049.
Article11. Boimer C, Birt CM. Preservative exposure and surgical outcomes in glaucoma patients: the PESO study. J Glaucoma. 2013; 22:730–735.12. Kim JW, Kang SH, Lee KW. Effect of ascorbic acid against the oxidative stress-induced cellular senescence in trabecular meshwork cells. J Korean Ophthalmol Soc. 2013; 54:490–495.
Article13. Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015; 21:1424–1435.
Article14. Liton PB, Challa P, Stinnett S, et al. Cellular senescence in the glaucomatous outflow pathway. Exp Gerontol. 2005; 40:745–748.
Article15. Park CH, Kim JW. Effect of advanced glycation end products on oxidative stress and senescence of trabecular meshwork cells. Korean J Ophthalmol. 2012; 26:123–131.
Article16. Lütjen-Drecoll E. Morphological changes in glaucomatous eyes and the role of TGFbeta2 for the pathogenesis of the disease. Exp Eye Res. 2005; 81:1–4.17. Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961; 25:585–621.
Article18. Toussaint O, Medrano EE, von Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol. 2000; 35:927–945.
Article19. Toussaint O, Royer V, Salmon M, Remacle J. Stress-induced premature senescence and tissue ageing. Biochem Pharmacol. 2002; 64:1007–1009.
Article20. Rohen JW, Lutjen-Drecoll E. Biology of the trabecular meshwork. In : Lutjen-Drecoll E, editor. Basic Aspects of Glaucoma Research, 1st ed. Stuttgard: Schattauer FK;1982. p. 141–166.21. Sherr CJ. Mammalian G1 cyclins. Cell. 1993; 73:1059–1065.
Article22. Xiong Y, Hannon GJ, Zhang H, et al. p21 is a universal inhibitor of cyclin kinases. Nature. 1993; 366:701–704.
Article23. Roninson IB. Tumor cell senescence in cancer treatment. Cancer Res. 2003; 63:2705–2715.24. Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007; 8:729–740.
Article25. Gu Z, Cao X, Jiang J, et al. Upregulation of p16INK4A promotes cellular senescence of bone marrow-derived mesenchymal stem cells from systemic lupus erythematosus patients. Cell Signal. 2012; 24:2307–2314.
Article26. Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965; 37:614–636.
Article27. Drummond-Barbosa D. Stem cells, their niches and the systemic environment: an aging network. Genetics. 2008; 180:1787–1797.
Article28. Chang BD, Broude EV, Dokmanovic M, et al. A senescence-like phenotype distinguishes tumor cells that undergo terminal proliferation arrest after exposure to anticancer agents. Cancer Res. 1999; 59:3761–3767.29. Freeman PD, Kahook MY. Preservatives in topical ophthalmic medications: historical and clinical perspectives. Expert Rev Ophthalmol. 2009; 4:59–64.
Article30. Pisella PJ, Debbasch C, Hamard P, et al. Conjunctival proinflammatory and proapoptotic effects of latanoprost and preserved and unpreserved timolol: an ex vivo and in vitro study. Invest Ophthalmol Vis Sci. 2004; 45:1360–1368.
Article31. Guenoun JM, Baudouin C, Rat P, et al. In vitro comparison of cytoprotective and antioxidative effects of latanoprost, travoprost, and bimatoprost on conjunctiva-derived epithelial cells. Invest Ophthalmol Vis Sci. 2005; 46:4594–4599.
Article32. Ammar DA, Noecker RJ, Kahook MY. Effects of benzalkonium chloride-preserved, polyquad-preserved, and sofZia-preserved topical glaucoma medications on human ocular epithelial cells. Adv Ther. 2010; 27:837–845.
Article33. Brignole-Baudouin F, Riancho L, Liang H, et al. In vitro comparative toxicology of polyquad-preserved and benzalkonium chloride-preserved travoprost/timolol fixed combination and latanoprost/timolol fixed combination. J Ocul Pharmacol Ther. 2011; 27:273–280.
Article34. Stevens AM, Kestelyn PA, De Bacquer D, Kestelyn PG. Benzalkonium chloride induces anterior chamber inflammation in previously untreated patients with ocular hypertension as measured by flare meter: a randomized clinical trial. Acta Ophthalmol. 2012; 90:e221–e224.
Article35. Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995; 92:9363–9367.
Article36. Samples JR, Binder PS, Nayak S. The effect of epinephrine and benzalkonium chloride on cultured corneal endothelial and trabecular meshwork cells. Exp Eye Res. 1989; 49:1–12.37. Aliouat-Denis CM, Dendouga N, Van den Wyngaert I, et al. p53-independent regulation of p21Waf1/Cip1 expression and senescence by Chk2. Mol Cancer Res. 2005; 3:627–634.
Article38. Beauséjour CM, Krtolica A, Galimi F, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003; 22:4212–4222.39. Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011; 192:547–556.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Bimatoprost on the Permeability of Trabecular Meshwork Cell Monolayer
- Effect of Hydrogen Peroxide-induced Oxidative Stress on the Senescence of Trabecular Meshwork Cells
- Effect of Benzalkonium, Mitomycin-C and Dexamethasone on Stress in Trabecular Meshwork Cells
- Effect of Ascorbic Acid Against the Oxidative Stress-Induced Cellular Senescence in Trabecular Meshwork Cells
- Effect of beta-adrenergics on the Survival and Production of Nitric Oxide in the Cultured Trabecular Meshwork Cells