Intest Res.
2017 Apr;15(2):174-181. 10.5217/ir.2017.15.2.174.
Parthenolide promotes apoptotic cell death and inhibits the migration and invasion of SW620 cells
- Affiliations
-
- 1Department of Internal Medicine, Research Institute of Clinical Medicine of Chonbuk National University Hospital, Jeonju, Korea. clickm@jbnu.ac.kr
- 2Biomedical Research Institute of Chonbuk National University Hospital, Chonbuk National University Medical School, Jeonju, Korea.
- KMID: 2425158
- DOI: http://doi.org/10.5217/ir.2017.15.2.174
Abstract
- BACKGROUND/AIMS
Parthenolide (PT), a principle component derived from feverfew (Tanacetum parthenium), is a promising anticancer agent and has been shown to promote apoptotic cell death in various cancer cells. In this study, we focused on its functional role in apoptosis, migration, and invasion of human colorectal cancer (CRC) cells.
METHODS
SW620 cells were employed as representative human CRC cells. We performed the MTT assay and cell cycle analysis to measure apoptotic cell death. The wound healing, Transwell migration, and Matrigel invasion assays were performed to investigate the effect of PT on cell migration/invasion. Western blotting was used to establish the signaling pathway of apoptosis and cell migration/invasion.
RESULTS
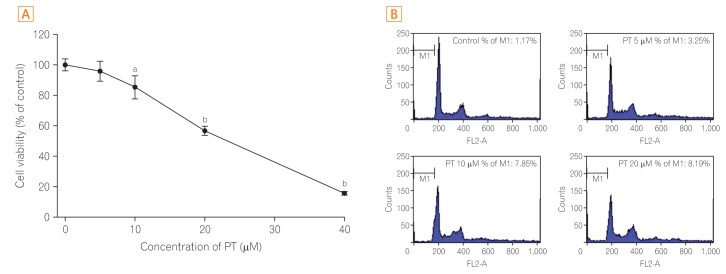
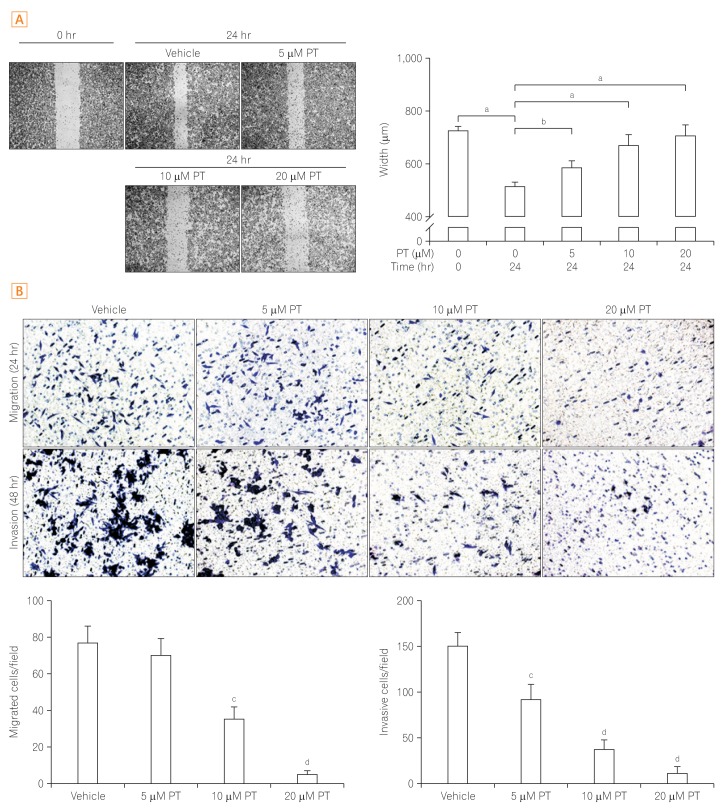
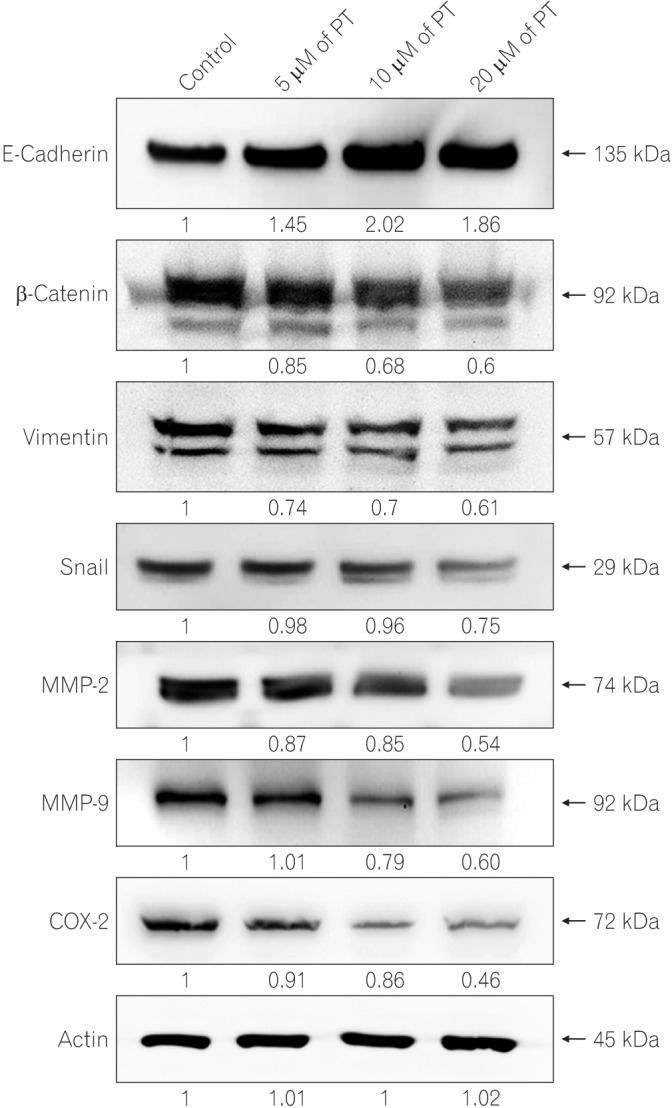
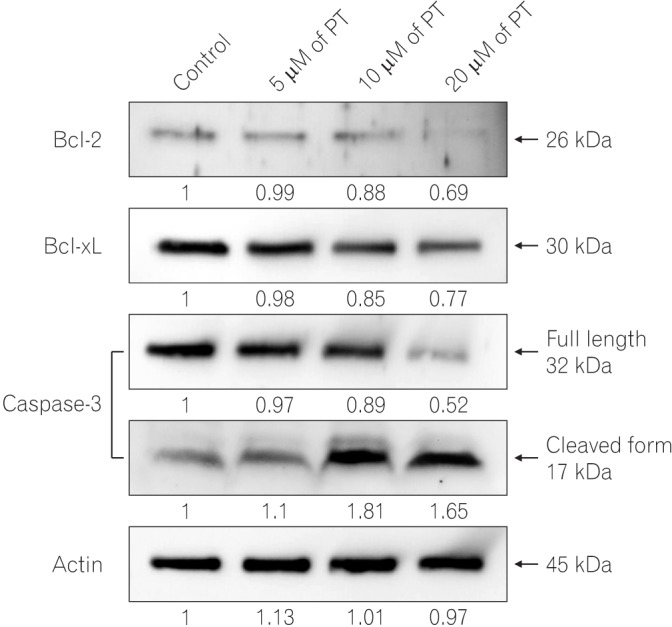
PT exerts antiproliferative effect and induces apoptotic cell death of SW620 cells. In addition, PT prevents cell migration and invasion in a dose-dependent manner. Moreover, PT markedly suppressed migration/invasion-related protein expression, including E-cadherin, β-catenin, vimentin, Snail, cyclooxygenase-2, matrix metalloproteinase-2 (MMP-2), and MMP-9 in SW620 cells. PT also inhibited the expression of antiapoptotic proteins (Bcl-2 and Bcl-xL) and activated apoptosis terminal factor (caspase-3) in a dose-dependent manner.
CONCLUSIONS
Our results suggest that PT is a potential novel therapeutic agent for aggressive CRC treatment.
Keyword
MeSH Terms
Figure
Reference
-
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90. PMID: 21296855.
Article2. Bosetti C, Levi F, Rosato V, et al. Recent trends in colorectal cancer mortality in Europe. Int J Cancer. 2011; 129:180–191. PMID: 20824701.
Article3. Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010; 116:544–573. PMID: 19998273.
Article4. Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014; 64:104–117. PMID: 24639052.
Article5. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012; 62:10–29. PMID: 22237781.
Article6. Bork PM, Schmitz ML, Kuhnt M, Escher C, Heinrich M. Sesquiterpene lactone containing Mexican Indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-kappaB. FEBS Lett. 1997; 402:85–90. PMID: 9013864.
Article7. Murphy JJ, Heptinstall S, Mitchell JR. Randomised double-blind placebo-controlled trial of feverfew in migraine prevention. Lancet. 1988; 2:189–192. PMID: 2899663.
Article8. Hehner SP, Heinrich M, Bork PM, et al. Sesquiterpene lactones specifically inhibit activation of NF-kappa B by preventing the degradation of I kappa B-alpha and I kappa B-beta. J Biol Chem. 1998; 273:1288–1297. PMID: 9430659.
Article9. Oka D, Nishimura K, Shiba M, et al. Sesquiterpene lactone parthenolide suppresses tumor growth in a xenograft model of renal cell carcinoma by inhibiting the activation of NF-kappaB. Int J Cancer. 2007; 120:2576–2581. PMID: 17290398.
Article10. Kishida Y, Yoshikawa H, Myoui A. Parthenolide, a natural inhibitor of nuclear factor-kappaB, inhibits lung colonization of murine osteosarcoma cells. Clin Cancer Res. 2007; 13:59–67. PMID: 17200339.
Article11. Zhang S, Ong CN, Shen HM. Critical roles of intracellular thiols and calcium in parthenolide-induced apoptosis in human colorectal cancer cells. Cancer Lett. 2004; 208:143–153. PMID: 15142672.
Article12. Wen J, You KR, Lee SY, Song CH, Kim DG. Oxidative stress-mediated apoptosis: the anticancer effect of the sesquiterpene lactone parthenolide. J Biol Chem. 2002; 277:38954–38964. PMID: 12151389.13. Kim SL, Trang KT, Kim SH, et al. Parthenolide suppresses tumor growth in a xenograft model of colorectal cancer cells by inducing mitochondrial dysfunction and apoptosis. Int J Oncol. 2012; 41:1547–1553. PMID: 22895542.
Article14. Kim SL, Liu YC, Park YR, et al. Parthenolide enhances sensitivity of colorectal cancer cells to TRAIL by inducing death receptor 5 and promotes TRAIL-induced apoptosis. Int J Oncol. 2015; 46:1121–1130. PMID: 25502339.
Article15. Kanthan R, Senger JL, Kanthan SC. Molecular events in primary and metastatic colorectal carcinoma: a review. Patholog Res Int. 2012; 2012:597497. PMID: 22997602.
Article16. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014; 15:178–196. PMID: 24556840.
Article17. Liu JW, Cai MX, Xin Y, et al. Parthenolide induces proliferation inhibition and apoptosis of pancreatic cancer cells in vitro. J Exp Clin Cancer Res. 2010; 29:108. PMID: 20698986.
Article18. Kwak SW, Park ES, Lee CS. Parthenolide induces apoptosis by activating the mitochondrial and death receptor pathways and inhibits FAK-mediated cell invasion. Mol Cell Biochem. 2014; 385:133–144. PMID: 24065392.
Article19. Toth M, Sohail A, Fridman R. Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol Biol. 2012; 878:121–135. PMID: 22674130.
Article20. Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005; 23:2840–2855. PMID: 15837998.
Article22. Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006; 172:973–981. PMID: 16567498.
Article23. Carlisi D, D'Anneo A, Angileri L, et al. Parthenolide sensitizes hepatocellular carcinoma cells to TRAIL by inducing the expression of death receptors through inhibition of STAT3 activation. J Cell Physiol. 2011; 226:1632–1641. PMID: 21413021.
Article24. Dai Y, Guzman ML, Chen S, et al. The NF (nuclear factor)-kappaB inhibitor parthenolide interacts with histone deacetylase inhibitors to induce MKK7/JNK1-dependent apoptosis in human acute myeloid leukaemia cells. Br J Haematol. 2010; 151:70–83. PMID: 20701602.
Article25. Nakshatri H, Rice SE, Bhat-Nakshatri P. Antitumor agent parthenolide reverses resistance of breast cancer cells to tumor necrosis factor-related apoptosis-inducing ligand through sustained activation of c-Jun N-terminal kinase. Oncogene. 2004; 23:7330–7344. PMID: 15286701.
Article26. Wu C, Chen F, Rushing JW, et al. Antiproliferative activities of parthenolide and golden feverfew extract against three human cancer cell lines. J Med Food. 2006; 9:55–61. PMID: 16579729.
Article27. Kim SL, Lee ST, Trang KT, et al. Parthenolide exerts inhibitory effects on angiogenesis through the downregulation of VEGF/VEGFRs in colorectal cancer. Int J Mol Med. 2014; 33:1261–1267. PMID: 24573421.
Article28. Amorim MH, Gil da Costa RM, Lopes C, Bastos MM. Sesquiterpene lactones: adverse health effects and toxicity mechanisms. Crit Rev Toxicol. 2013; 43:559–579. PMID: 23875764.
Article29. Chadwick M, Trewin H, Gawthrop F, Wagstaff C. Sesquiterpenoids lactones: benefits to plants and people. Int J Mol Sci. 2013; 14:12780–12805. PMID: 23783276.
Article30. Chiu KY, Wu CC, Chia CH, Hsu SL, Tzeng YM. Inhibition of growth, migration and invasion of human bladder cancer cells by antrocin, a sesquiterpene lactone isolated from Antrodia cinnamomea, and its molecular mechanisms. Cancer Lett. 2016; 373:174–184. PMID: 26679052.
Article31. Fu J, Ke X, Tan S, et al. The natural compound codonolactone attenuates TGF-beta1-mediated epithelial-to-mesenchymal transition and motility of breast cancer cells. Oncol Rep. 2016; 35:117–126. PMID: 26549400.
Article32. Tabata K, Nishimura Y, Takeda T, Kurita M, Uchiyama T, Suzuki T. Sesquiterpene lactones derived from Saussurea lappa induce apoptosis and inhibit invasion and migration in neuroblastoma cells. J Pharmacol Sci. 2015; 127:397–403. PMID: 25953266.
Article33. Qin G, Xu F, Qin T, et al. Palbociclib inhibits epithelial-mesenchymal transition and metastasis in breast cancer via c-Jun/COX-2 signaling pathway. Oncotarget. 2015; 6:41794–41808. PMID: 26540629.
Article34. Li ZL, Ye SB, OuYang LY, et al. COX-2 promotes metastasis in nasopharyngeal carcinoma by mediating interactions between cancer cells and myeloid-derived suppressor cells. Oncoimmunology. 2015; 4:e1044712. PMID: 26451317.
Article35. Wang KH, Kao AP, Chang CC, Lin TC, Kuo TC. Bisphenol Ainduced epithelial to mesenchymal transition is mediated by cyclooxygenase-2 up-regulation in human endometrial carcinoma cells. Reprod Toxicol. 2015; 58:229–233. PMID: 26546977.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Parthenolide inhibits transforming growth factor β1-induced epithelial-mesenchymal transition in colorectal cancer cells
- Balsalazide Potentiates Parthenolide-Mediated Inhibition of Nuclear Factor-kappaB Signaling in HCT116 Human Colorectal Cancer Cells
- Kinesin superfamily member 15 knockdown inhibits cell proliferation, migration, and invasion in nasopharyngeal carcinoma
- Synergistic Effect of Parthenolide in Combination with 5-Fluorouracil in SW480 Cells
- Inhibition of Cell Migration by Corticotropin-Releasing Hormone (CRH) in Human Natural Killer Cell Line, NK-92MI