Korean J Physiol Pharmacol.
2023 Sep;27(5):457-470. 10.4196/kjpp.2023.27.5.457.
Kinesin superfamily member 15 knockdown inhibits cell proliferation, migration, and invasion in nasopharyngeal carcinoma
- Affiliations
-
- 1Guangzhou Municipal and Guangdong Provincial Key Laboratory of Molecular Target & Clinical Pharmacology, the NMPA and State Key Laboratory of Respiratory Disease, School of Pharmaceutical Sciences and the Fifth Affiliated Hospital, Guangzhou Medical University, Guangzhou 511436, China
- 2Laboratory Animal Centre, Guangzhou Medical University, Guangzhou 511436, China
- 3The Second Affiliated Hospital, Guangzhou Medical University, Guangzhou 510260, China
- KMID: 2545536
- DOI: http://doi.org/10.4196/kjpp.2023.27.5.457
Abstract
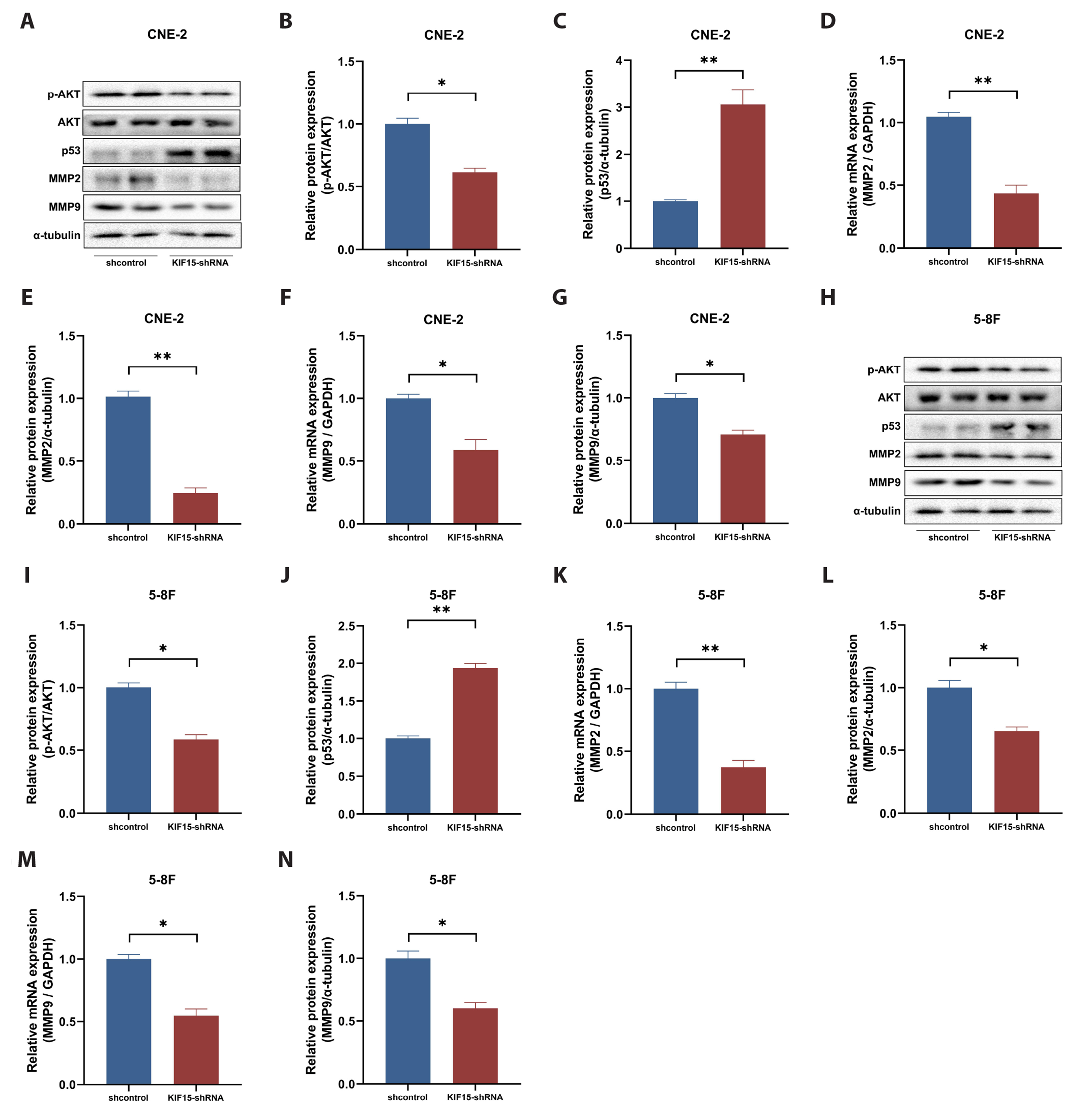
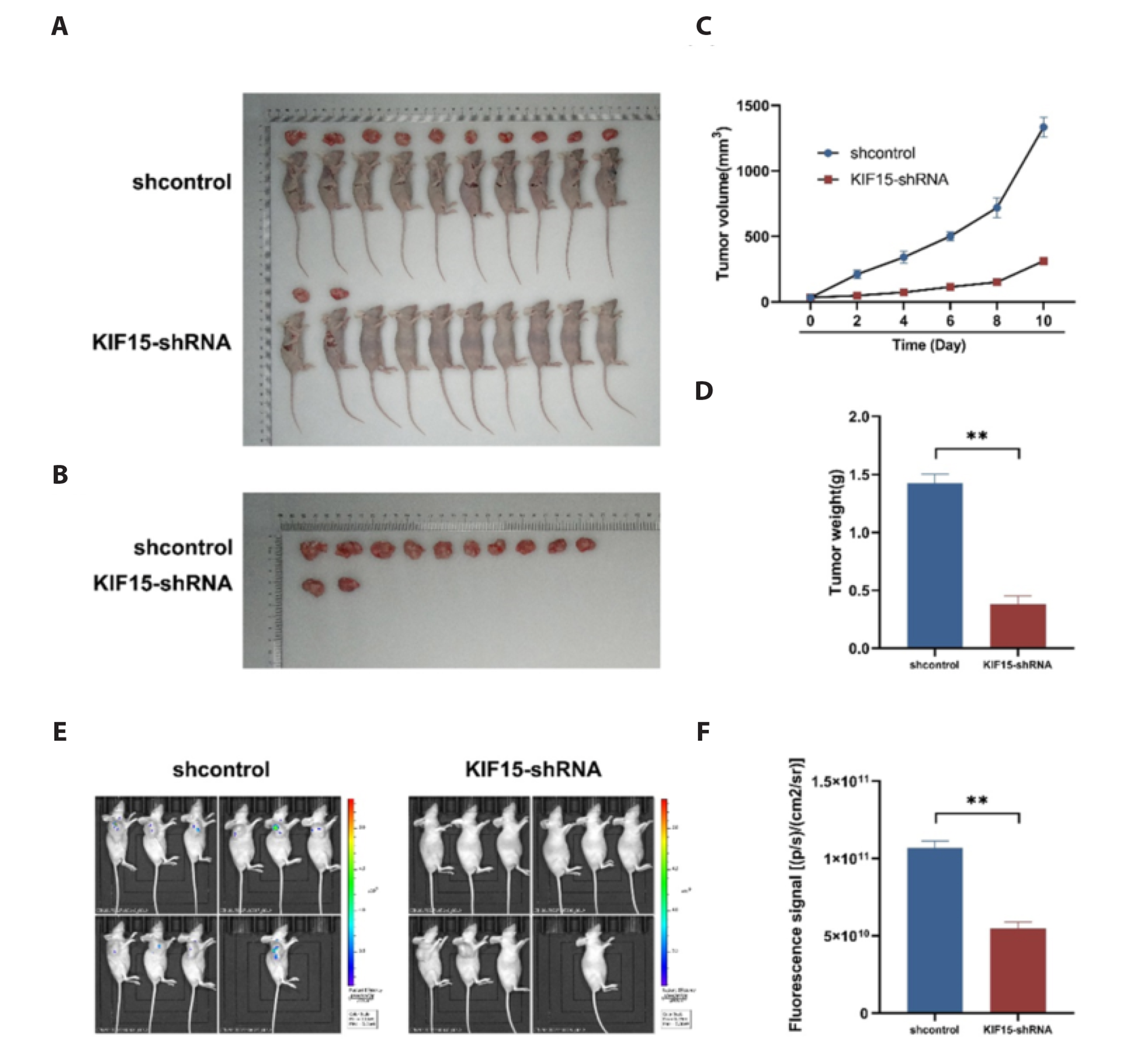
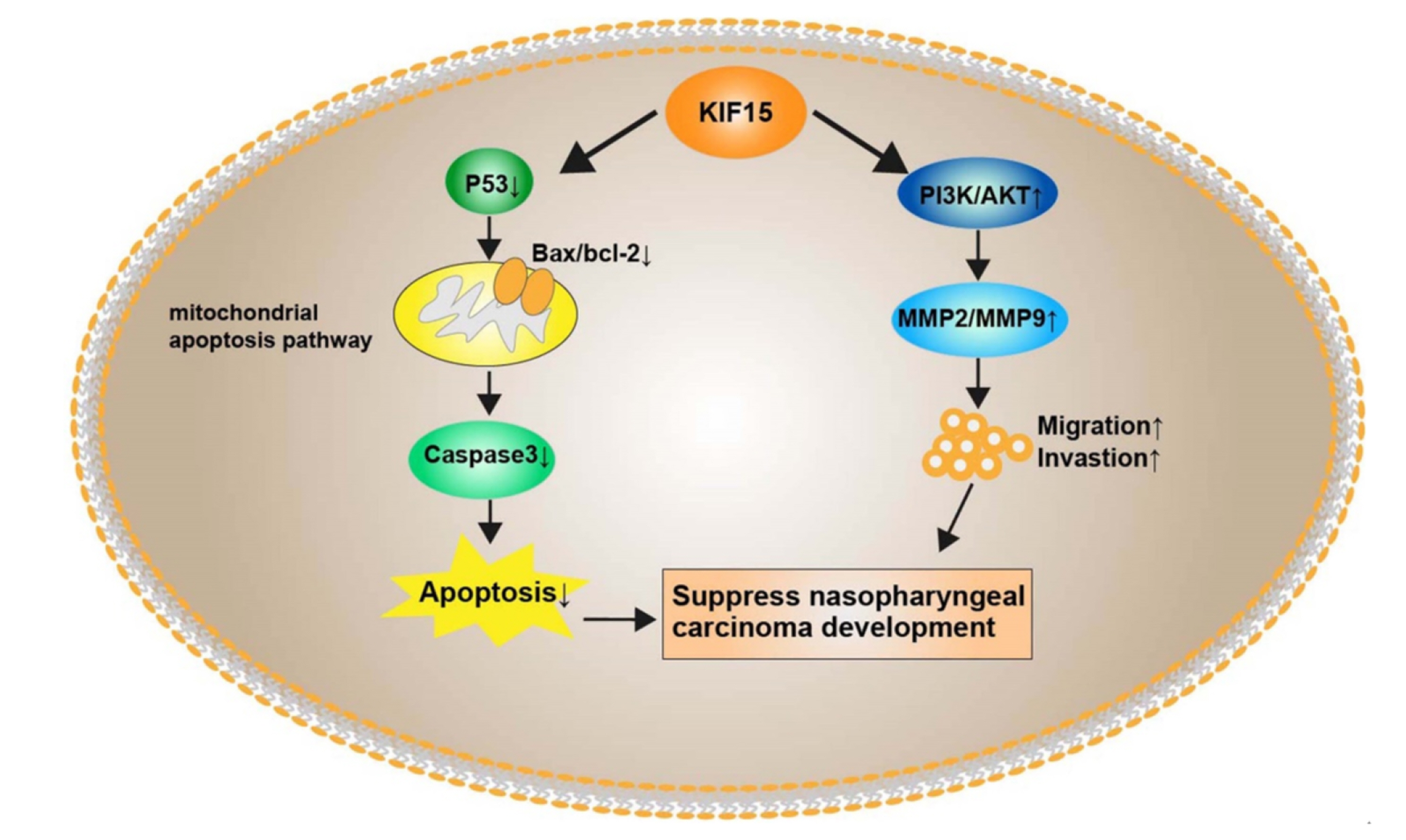
- The aim of this study was to investigate the role of kinesin superfamily member 15 (KIF15) in nasopharyngeal carcinogenesis (NPC) and explore its underlying mechanisms. We employed various assays, including the CCK-8 assay, flow cytometry, the Transwell and scratch assay, Western blotting, and nude mice transplantation tumor, to investigate the impact of KIF15 on NPC. Our findings demonstrate that KIF15 plays a critical role in the proliferation, apoptosis, migration, and invasion of NPC cells. Furthermore, we discovered that silencing KIF15 inhibits cell proliferation, migration, and invasion while promoting apoptosis, and that KIF15's effect on NPC cell growth is mediated through the PI3K/AKT and P53 signaling pathways. Additionally, we showed that KIF15 promotes nasopharyngeal cancer cell growth in vivo. Our study sheds light on the significance of KIF15 in NPC by revealing that KIF15 knockdown inhibits NPC cell growth through the regulation of AKT-related signaling pathways. These findings suggest that KIF15 represents a promising therapeutic target for the prevention and treatment of NPC.
Figure
Reference
-
1. Tang SQ, Mao YP, Xu C, Guo R, Li WF, Tang LL, Sun Y, Ma J. 2020; The evolution of the nasopharyngeal carcinoma staging system over a 10-year period: implications for future revisions. Chin Med J (Engl). 133:2044–2053. DOI: 10.1097/CM9.0000000000000978. PMID: 32810045. PMCID: PMC7478675. PMID: 45b20d7da1104cda981fe16dd2ef952e.
Article2. Hung CC, Tu MY, Chien TW, Lin CY, Chow JC, Chou W. 2023; The model of descriptive, diagnostic, predictive, and prescriptive analytics on 100 top-cited articles of nasopharyngeal carcinoma from 2013 to 2022: bibliometric analysis. Medicine (Baltimore). 102:e32824. DOI: 10.1097/MD.0000000000032824. PMID: 36820592. PMCID: PMC9907932.
Article3. Pontoriero F, Silverman AM, Pascasio JM, Bajaj R. 2020; Nonkeratinizing nasopharyngeal carcinoma, undifferentiated type with trisomy 2: a case report and short review of cytogenetic and molecular literature. Pediatr Dev Pathol. 23:448–452. DOI: 10.1177/1093526620945861. PMID: 32755442.
Article4. Lam WKJ, Chan JYK. 2018; Recent advances in the management of nasopharyngeal carcinoma. F1000Res. 7:F1000 Faculty Rev-1829. DOI: 10.12688/f1000research.15066.1. PMID: 30519454. PMCID: PMC6249636. PMID: 8086e0a2c60c441698858e8bc2691c39.
Article5. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. 2019; Nasopharyngeal carcinoma. Lancet. 394:64–80. DOI: 10.1016/S0140-6736(19)30956-0. PMID: 31178151.
Article6. Lee HM, Okuda KS, González FE, Patel V. 2019; Current perspectives on nasopharyngeal carcinoma. Adv Exp Med Biol. 1164:11–34. DOI: 10.1007/978-3-030-22254-3_2. PMID: 31576537.
Article7. Lee YT, Tan YJ, Oon CE. 2018; Molecular targeted therapy: treating cancer with specificity. Eur J Pharmacol. 834:188–196. DOI: 10.1016/j.ejphar.2018.07.034. PMID: 30031797.
Article8. Hirokawa N, Noda Y, Tanaka Y, Niwa S. 2009; Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 10:682–696. DOI: 10.1038/nrm2774. PMID: 19773780.
Article9. Huo D, Yang H, Huang JD, Cai JP, Cui J. 2021; Roles of kinesin superfamily proteins in colorectal cancer carcinogenesis (Review). Oncol Rep. 46:121. DOI: 10.3892/or.2021.8072. PMID: 33955521.
Article10. Niwa S. 2015; Kinesin superfamily proteins and the regulation of microtubule dynamics in morphogenesis. Anat Sci Int. 90:1–6. DOI: 10.1007/s12565-014-0259-5. PMID: 25347970.
Article11. Wang Z, Chen M, Fang X, Hong H, Yao Y, Huang H. 2021; KIF15 is involved in development and progression of Burkitt lymphoma. Cancer Cell Int. 21:261. DOI: 10.1186/s12935-021-01967-z. PMID: 33985517. PMCID: PMC8117549. PMID: dbca50a309e04f1389cba3241fd2e9fd.
Article12. Sun RF, He N, Zhang GY, Yu ZY, Li LS, Ma ZJ, Jiao ZY. 2023; Combined inhibition of KIF11 and KIF15 as an effective therapeutic strategy for gastric cancer. Curr Cancer Drug Targets. 23:293–306. DOI: 10.2174/1568009622666220616122846. PMID: 35713129.13. Klejnot M, Falnikar A, Ulaganathan V, Cross RA, Baas PW, Kozielski F. 2014; The crystal structure and biochemical characterization of Kif15: a bifunctional molecular motor involved in bipolar spindle formation and neuronal development. Acta Crystallogr D Biol Crystallogr. 70(Pt 1):123–133. DOI: 10.1107/S1399004713028721. PMID: 24419385. PMCID: PMC3919264.
Article14. Gao X, Zhu L, Lu X, Wang Y, Li R, Jiang G. 2020; KIF15 contributes to cell proliferation and migration in breast cancer. Hum Cell. 33:1218–1228. DOI: 10.1007/s13577-020-00392-0. PMID: 32578050.
Article15. Sun YF, Wu HL, Shi RF, Chen L, Meng C. 2020; KIF15 promotes proliferation and growth of hepatocellular carcinoma. Anal Cell Pathol (Amst). 2020:6403012. DOI: 10.1155/2020/6403012. PMID: 32318326. PMCID: PMC7157793. PMID: 56c6e1dbeba04ef68252ee05e62e5a77.
Article16. Gao L, Zhang W, Zhang J, Liu J, Sun F, Liu H, Hu J, Wang X, Wang X, Su P, Chen S, Qu S, Shi B, Xiong X, Chen W, Dong X, Han B. 2021; KIF15-mediated stabilization of AR and AR-V7 contributes to enzalutamide resistance in prostate cancer. Cancer Res. 81:1026–1039. DOI: 10.1158/0008-5472.CAN-20-1965. PMID: 33277366.
Article17. Perri F, Della Vittoria Scarpati G, Caponigro F, Ionna F, Longo F, Buonopane S, Muto P, Di Marzo M, Pisconti S, Solla R. 2019; Management of recurrent nasopharyngeal carcinoma: current perspectives. Onco Targets Ther. 12:1583–1591. DOI: 10.2147/OTT.S188148. PMID: 30881013. PMCID: PMC6396653.18. Vasudevan HN, Yom SS. 2021; Nasopharyngeal carcinoma and its association with Epstein-Barr virus. Hematol Oncol Clin North Am. 35:963–971. DOI: 10.1016/j.hoc.2021.05.007. PMID: 34187713.
Article19. Yu Y, Feng YM. 2010; The role of kinesin family proteins in tumorigenesis and progression: potential biomarkers and molecular targets for cancer therapy. Cancer. 116:5150–5160. DOI: 10.1002/cncr.25461. PMID: 20661912.
Article20. Singel SM, Cornelius C, Zaganjor E, Batten K, Sarode VR, Buckley DL, Peng Y, John GB, Li HC, Sadeghi N, Wright WE, Lum L, Corson TW, Shay JW. 2014; KIF14 promotes AKT phosphorylation and contributes to chemoresistance in triple-negative breast cancer. Neoplasia. 16:247–256.e2. DOI: 10.1016/j.neo.2014.03.008. PMID: 24784001. PMCID: PMC4094827. PMID: 6c64beef889741d38f469bb388b70ed5.
Article21. Shimo A, Tanikawa C, Nishidate T, Lin ML, Matsuda K, Park JH, Ueki T, Ohta T, Hirata K, Fukuda M, Nakamura Y, Katagiri T. 2008; Involvement of kinesin family member 2C/mitotic centromere-associated kinesin overexpression in mammary carcinogenesis. Cancer Sci. 99:62–70. DOI: 10.1111/j.1349-7006.2007.00635.x. PMID: 17944972.
Article22. Nagahara M, Nishida N, Iwatsuki M, Ishimaru S, Mimori K, Tanaka F, Nakagawa T, Sato T, Sugihara K, Hoon DS, Mori M. 2011; Kinesin 18A expression: clinical relevance to colorectal cancer progression. Int J Cancer. 129:2543–2552. DOI: 10.1002/ijc.25916. PMID: 21213216.
Article23. Ding L, Li B, Yu X, Li Z, Li X, Dang S, Lv Q, Wei J, Sun H, Chen H, Liu M, Li G. 2020; KIF15 facilitates gastric cancer via enhancing proliferation, inhibiting apoptosis, and predict poor prognosis. Cancer Cell Int. 20:125. DOI: 10.1186/s12935-020-01199-7. PMID: 32322172. PMCID: PMC7160940. PMID: 477878391acb4aaebcaecb0a53c96a89.
Article24. Zheng S, Tang D, Wang X, Liu C, Zuo N, Yan R, Wu C, Ma J, Wang C, Xu H, He Y, Liu D, Liu S. 2022; Kif15 is required in the development of auditory system using zebrafish as a model. Front Mol Neurosci. 15:844568. DOI: 10.3389/fnmol.2022.844568. PMID: 35370541. PMCID: PMC8971910. PMID: 459e99e04b934a66bd00f63a2bbe352f.
Article25. Xu M, Liu D, Dong Z, Wang X, Wang X, Liu Y, Baas PW, Liu M. 2014; Kinesin-12 influences axonal growth during zebrafish neural development. Cytoskeleton (Hoboken). 71:555–563. DOI: 10.1002/cm.21193. PMID: 25250533. PMCID: PMC4236235.
Article26. Wang J, Wang D, Fei Z, Feng D, Zhang B, Gao P, Hu G, Li W, Huang X, Chen D, Ding X, Wu W. 2021; KIF15 knockdown suppresses gallbladder cancer development. Eur J Cell Biol. 100:151182. DOI: 10.1016/j.ejcb.2021.151182. PMID: 34781077.
Article27. Wade M, Li YC, Wahl GM. 2013; MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat Rev Cancer. 13:83–96. DOI: 10.1038/nrc3430. PMID: 23303139. PMCID: PMC4161369.
Article28. Blagih J, Buck MD, Vousden KH. 2020; p53, cancer and the immune response. J Cell Sci. 133:jcs237453. DOI: 10.1242/jcs.237453. PMID: 32144194.
Article29. Kale J, Osterlund EJ, Andrews DW. 2018; BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ. 25:65–80. DOI: 10.1038/cdd.2017.186. PMID: 29149100. PMCID: PMC5729540.
Article30. Weng C, Chen Y, Wu Y, Liu X, Mao H, Fang X, Li B, Wang L, Guan M, Liu G, Lu L, Yuan Y. 2019; Silencing UBE4B induces nasopharyngeal carcinoma apoptosis through the activation of caspase3 and p53. Onco Targets Ther. 12:2553–2561. DOI: 10.2147/OTT.S196132. PMID: 31040698. PMCID: PMC6459139.31. Wang Z, Liao K, Zuo W, Liu X, Qiu Z, Gong Z, Liu C, Zeng Q, Qian Y, Jiang L, Bu Y, Hong S, Hu G. 2017; Depletion of NFBD1/MDC1 induces apoptosis in nasopharyngeal carcinoma cells through the p53-ROS-mitochondrial pathway. Oncol Res. 25:123–136. DOI: 10.3727/096504016X14732772150226. PMID: 28081741. PMCID: PMC7840771.
Article32. Jiang S, Huang C, Zheng G, Yi W, Wu B, Tang J, Liu X, Huang B, Wu D, Yan T, Li M, Wan C, Cai Y. 2022; EGCG inhibits proliferation and induces apoptosis through downregulation of SIRT1 in nasopharyngeal carcinoma cells. Front Nutr. 9:851972. DOI: 10.3389/fnut.2022.851972. PMID: 35548580. PMCID: PMC9084317. PMID: 6b11df6f0e6347b89f84c58a0682b4e7.
Article33. Hernández Borrero LJ, El-Deiry WS. 2021; Tumor suppressor p53: biology, signaling pathways, and therapeutic targeting. Biochim Biophys Acta Rev Cancer. 1876:188556. DOI: 10.1016/j.bbcan.2021.188556. PMID: 33932560. PMCID: PMC8730328.
Article34. Junttila MR, Evan GI. 2009; p53--a Jack of all trades but master of none. Nat Rev Cancer. 9:821–829. DOI: 10.1038/nrc2728. PMID: 19776747.35. Moreira J, Almeida J, Saraiva L, Cidade H, Pinto M. 2021; Chalcones as promising antitumor agents by targeting the p53 pathway: an overview and new insights in drug-likeness. Molecules. 26:3737. DOI: 10.3390/molecules26123737. PMID: 34205272. PMCID: PMC8233907. PMID: 6b9d17e5acf149adb7d23cb2cbf99477.
Article36. Kruse JP, Gu W. 2009; Modes of p53 regulation. Cell. 137:609–622. DOI: 10.1016/j.cell.2009.04.050. PMID: 19450511. PMCID: PMC3737742.
Article37. Mayo LD, Donner DB. 2001; A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci U S A. 98:11598–11603. DOI: 10.1073/pnas.181181198. PMID: 11504915. PMCID: PMC58775.
Article38. Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung MC. 2001; HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 3:973–982. Erratum in: Nat Cell Biol. 2002; 4:736. DOI: 10.1038/ncb1101-973. PMID: 11715018.
Article39. Zanotelli MR, Zhang J, Reinhart-King CA. 2021; Mechanoresponsive metabolism in cancer cell migration and metastasis. Cell Metab. 33:1307–1321. DOI: 10.1016/j.cmet.2021.04.002. PMID: 33915111. PMCID: PMC9015673.
Article40. Liang W, Shi J, Xia H, Wei X. 2021; A novel ruthenium-fluvastatin complex downregulates SNCG expression to modulate breast carcinoma cell proliferation and apoptosis via activating the PI3K/Akt/mTOR/VEGF/MMP9 pathway. Oxid Med Cell Longev. 2021:5537737. DOI: 10.1155/2021/5537737. PMID: 34221232. PMCID: PMC8221895.
Article41. Zhu W, Wu X, Yang B, Yao X, Cui X, Xu P, Chen X. 2019; miR-188-5p regulates proliferation and invasion via PI3K/Akt/MMP-2/9 signaling in keloids. Acta Biochim Biophys Sin (Shanghai). 51:185–196. Erratum in: Acta Biochim Biophys Sin (Shanghai). 2019;51:980. DOI: 10.1093/abbs/gmy165. PMID: 30668826.
Article42. Zhu Y, Yan L, Zhu W, Song X, Yang G, Wang S. 2019; MMP2/3 promote the growth and migration of laryngeal squamous cell carcinoma via PI3K/Akt-NF-κB-mediated epithelial-mesenchymal transformation. J Cell Physiol. 234:15847–15855. DOI: 10.1002/jcp.28242. PMID: 30714134.
Article43. Li HL, Deng NH, He XS, Li YH. 2022; Small biomarkers with massive impacts: PI3K/AKT/mTOR signalling and microRNA crosstalk regulate nasopharyngeal carcinoma. Biomark Res. 10:52. DOI: 10.1186/s40364-022-00397-x. PMID: 35883139. PMCID: PMC9327212. PMID: 5e5616c222d647368dacc772e1aca5c1.
Article44. Xie X, Xiong G, Chen W, Fu H, Li M, Cui X. 2020; FOXD3 inhibits cell proliferation, migration, and invasion in nasopharyngeal carcinoma through regulation of the PI3K-Akt pathway. Biochem Cell Biol. 98:653–660. DOI: 10.1139/bcb-2020-0011. PMID: 32459973.
Article45. Fan X, Xie X, Yang M, Wang Y, Wu H, Deng T, Weng X, Wen W, Nie G. 2021; YBX3 mediates the metastasis of nasopharyngeal carcinoma via PI3K/AKT signaling. Front Oncol. 11:617621. DOI: 10.3389/fonc.2021.617621. PMID: 33816248. PMCID: PMC8010247. PMID: 358fd794b7514d329b00141c1817b77b.
Article46. Ho HC, Huang CC, Lu YT, Yeh CM, Ho YT, Yang SF, Hsin CH, Lin CW. 2019; Epigallocatechin-3-gallate inhibits migration of human nasopharyngeal carcinoma cells by repressing MMP-2 expression. J Cell Physiol. 234:20915–20924. DOI: 10.1002/jcp.28696. PMID: 31012106.
Article47. Luo Y, Zhang B, Xu L, Li M, Wu J, Zhou Y, Li Y. 2022; Downregulation of KIF15 inhibits the tumorigenesis of non-small-cell lung cancer via inactivating Raf/MEK/ERK signaling. Histol Histopathol. 37:269–285. DOI: 10.14670/HH-18-408. PMID: 34908156.48. Ge W, Chen Y, Guo Y, Zhao D, Mu L, Zhang K, Zhuo W. 2021; KIF15 upregulation promotes leiomyosarcoma cell growth via promoting USP15-mediated DEK deubiquitylation. Biochem Biophys Res Commun. 570:117–124. DOI: 10.1016/j.bbrc.2021.07.042. PMID: 34280614.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Alisol A Inhibited the Proliferation, Migration, and Invasion of Nasopharyngeal Carcinoma Cells by Inhibiting the Hippo Signaling Pathway
- CDK1 promotes the phosphorylation of KIFC1 to regulate the tumorgenicity of endometrial carcinoma
- Effect of BMI1 Knockdown on Cell Proliferation, Apoptosis, Invasiveness, and Migration of U251 Glioma Cells
- Kinesin superfamily protein member 4 (KIF4) is localized to midzone and midbody in dividing cells
- LETM1 Promotes Gastric Cancer Cell Proliferation, Migration, and Invasion via the PI3K/Akt Signaling Pathway