Intest Res.
2015 Jul;13(3):233-241. 10.5217/ir.2015.13.3.233.
Balsalazide Potentiates Parthenolide-Mediated Inhibition of Nuclear Factor-kappaB Signaling in HCT116 Human Colorectal Cancer Cells
- Affiliations
-
- 1Department of Internal Medicine, Medical School of Chonbuk National University, Jeonju, Korea. clickm@jbnu.ac.kr
- 2Colon Carcinogenesis and Inflammation Laboratory, Biomedical Research Institute of Chonbuk National University Hospital, Jeonju, Korea.
- KMID: 2174471
- DOI: http://doi.org/10.5217/ir.2015.13.3.233
Abstract
- BACKGROUND/AIMS
Balsalazide is an anti-inflammatory drug used in the treatment of inflammatory bowel disease. Balsalazide can reduce inflammatory responses via several mechanisms, including inhibition of nuclear factor-kappaB (NF-kappaB) activity. Parthenolide (PT) inhibits NF-kappaB and exerts promising anticancer effects by promoting apoptosis. The present investigated the antitumor effects of balsalazide, combined with PT, on NF-kappaB in a representative human colorectal carcinoma cell line, HCT116.
METHODS
We counted cells and conducted annexin-V assays and cell cycle analysis to measure apoptotic cell death. Western blotting was used investigate the levels of proteins involved in apoptosis.
RESULTS
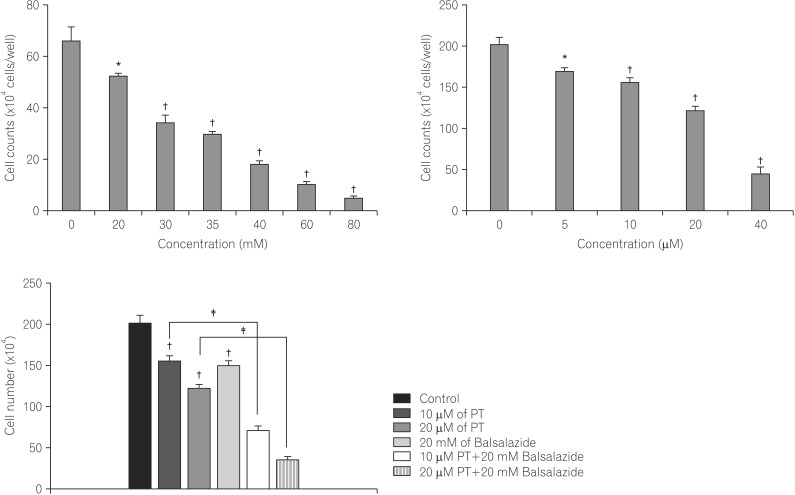
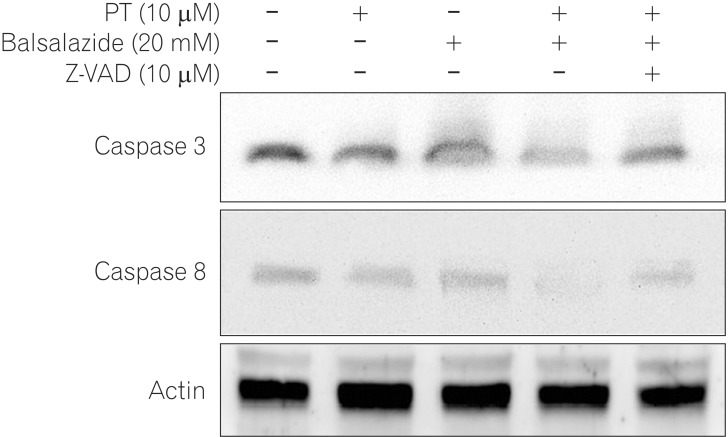
PT and balsalazide produced synergistic anti-proliferative effects and induced apoptotic cell death. The combination of balsalazide and PT markedly suppressed nuclear translocation of the NF-kappaB p65 subunit and the phosphorylation of inhibitor of NF-kappaB. Moreover, PT and balsalazide dramatically enhanced NF-kappaB p65 phosphorylation. Apoptosis, through the mitochondrial pathway, was confirmed by detecting effects on Bcl-2 family members, cytochrome c release, and activation of caspase-3 and -8.
CONCLUSIONS
Combination treatment with PT and balsalazide may offer an effective strategy for the induction of apoptosis in HCT116 cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Sandborn WJ, Hanauer SB. Systematic review: the pharmacokinetic profiles of oral mesalazine formulations and mesalazine pro-drugs used in the management of ulcerative colitis. Aliment Pharmacol Ther. 2003; 17:29–42. PMID: 12492730.
Article2. Sutherland L, Macdonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2006; CD000543. DOI: 10.1002/14651858.CD000543.pub2. Published online 19 April 2006. PMID: 16625536.
Article3. Feagan BG, Macdonald JK. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012; 10:CD000544. DOI: 10.1002/14651858.CD000544.pub3. Published online 17 October 2012. PMID: 23076890.
Article4. Shanahan F, Niederlehner A, Carramanzana N, Anton P. Sulfasalazine inhibits the binding of TNF alpha to its receptor. Immunopharmacology. 1990; 20:217–224. PMID: 1981213.
Article5. Bantel H, Berg C, Vieth M, Stolte M, Kruis W, Schulze-Osthoff K. Mesalazine inhibits activation of transcription factor NF-κB in inflamed mucosa of patients with ulcerative colitis. Am J Gastroenterol. 2000; 95:3452–3457. PMID: 11151876.
Article6. Bork PM, Schmitz ML, Kuhnt M, Escher C, Heinrich M. Sesquiterpene lactone containing Mexican Indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-κB. FEBS Lett. 1997; 402:85–90. PMID: 9013864.
Article7. Heinrich M, Robles M, West JE, Ortiz de Montellano BR, Rodriguez E. Ethnopharmacology of Mexican asteraceae (Compositae). Annu Rev Pharmacol Toxicol. 1998; 38:539–565. PMID: 9597165.
Article8. Murphy JJ, Heptinstall S, Mitchell JR. Randomised double-blind placebo-controlled trial of feverfew in migraine prevention. Lancet. 1988; 2:189–192. PMID: 2899663.
Article9. Schinella GR, Giner RM, Recio MC, Mordujovich de Buschiazzo P, Ríos JL, Máñez S. Anti-inflammatory effects of South American Tanacetum vulgare. J Pharm Pharmacol. 1998; 50:1069–1074. PMID: 9811170.10. Hehner SP, Heinrich M, Bork PM, et al. Sesquiterpene lactones specifically inhibit activation of NF-κB-α by preventing the degradation of IκB-α and IκB-β. J Biol Chem. 1998; 273:1288–1297. PMID: 9430659.
Article11. Oka D, Nishimura K, Shiba M, et al. Sesquiterpene lactone parthenolide suppresses tumor growth in a xenograft model of renal cell carcinoma by inhibiting the activation of NF-κB. Int J Cancer. 2007; 120:2576–2581. PMID: 17290398.
Article12. Kim SL, Trang KT, Jeon BJ, et al. Synergistic effect of parthenolide in combination with 5-fluorouracil in SW480 cells. Intest Res. 2012; 10:357–364.
Article13. Trang KT, Kim SL, Park SB, et al. Parthenolide sensitizes human colorectal cancer cells to tumor necrosis factor-related apoptosis-inducing ligand through mitochondrial and caspase dependent pathway. Intest Res. 2014; 12:34–41. PMID: 25349561.
Article14. Zhang S, Ong CN, Shen HM. Critical roles of intracellular thiols and calcium in parthenolide-induced apoptosis in human colorectal cancer cells. Cancer Lett. 2004; 208:143–153. PMID: 15142672.
Article15. Wen J, You KR, Lee SY, Song CH, Kim DG. Oxidative stress-mediated apoptosis. The anticancer effect of the sesquiterpene lactone parthenolide. J Biol Chem. 2002; 277:38954–38964. PMID: 12151389.16. Vermeulen L, De Wilde G, Notebaert S, Vanden Berghe W, Haegeman G. Regulation of the transcriptional activity of the nuclear factor-κB p65 subunit. Biochem Pharmacol. 2002; 64:963–970. PMID: 12213593.
Article17. Slee EA, Adrain C, Martin SJ. Serial killers: ordering caspase activation events in apoptosis. Cell Death Differ. 1999; 6:1067–1074. PMID: 10578175.
Article18. Yao J, Jiang Z, Duan W, et al. Involvement of mitochondrial pathway in triptolide-induced cytotoxicity in human normal liver L-02 cells. Biol Pharm Bull. 2008; 31:592–597. PMID: 18379047.
Article19. Rhodes JM, Campbell BJ. Inflammation and colorectal cancer: IBD-associated and sporadic cancer compared. Trends Mol Med. 2002; 8:10–16. PMID: 11796261.
Article20. Pinczowski D, Ekbom A, Baron J, Yuen J, Adami HO. Risk factors for colorectal cancer in patients with ulcerative colitis: a case-control study. Gastroenterology. 1994; 107:117–120. PMID: 7912678.
Article21. Stolfi C, Pellegrini R, Franze E, Pallone F, Monteleone G. Molecular basis of the potential of mesalazine to prevent colorectal cancer. World J Gastroenterol. 2008; 14:4434–4439. PMID: 18680220.
Article22. Bernstein CN, Nugent Z, Blanchard JF. 5-aminosalicylate is not chemoprophylactic for colorectal cancer in IBD: a population based study. Am J Gastroenterol. 2011; 106:731–736. PMID: 21407180.
Article23. Kim SL, Trang KT, Kim SH, et al. Parthenolide suppresses tumor growth in a xenograft model of colorectal cancer cells by inducing mitochondrial dysfunction and apoptosis. Int J Oncol. 2012; 41:1547–1553. PMID: 22895542.
Article24. Kim SL, Lee ST, Trang KT, et al. Parthenolide exerts inhibitory effects on angiogenesis through the downregulation of VEGF/VEGFRs in colorectal cancer. Int J Mol Med. 2014; 33:1261–1267. PMID: 24573421.
Article25. Kim SL, Kim SH, Trang KT, et al. Synergistic antitumor effect of 5-fluorouracil in combination with parthenolide in human colorectal cancer. Cancer Lett. 2013; 335:479–486. PMID: 23507557.
Article26. Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009; 15:103–113. PMID: 19185845.
Article27. Zhao ZJ, Xiang JY, Liu L, Huang XL, Gan HT. Parthenolide, an inhibitor of the nuclear factor-κB pathway, ameliorates dextran sulfate sodium-induced colitis in mice. Int Immunopharmacol. 2012; 12:169–174. PMID: 22155740.
Article28. Richmond A. NF-κB, chemokine gene transcription and tumour growth. Nat Rev Immunol. 2002; 2:664–674. PMID: 12209135.
Article29. Hengartner MO. The biochemistry of apoptosis. Nature. 2000; 407:770–776. PMID: 11048727.
Article30. Chiarugi V, Magnelli L, Cinelli M, Basi G. Apoptosis and the cell cycle. Cell Mol Biol Res. 1994; 40:603–612. PMID: 7787878.31. Baker SJ, Reddy EP. Modulation of life and death by the TNF receptor superfamily. Oncogene. 1998; 17:3261–3270. PMID: 9916988.
Article32. Reed JC. Double identity for proteins of the Bcl-2 family. Nature. 1997; 387:773–776. PMID: 9194558.
Article33. Estrov Z, Manna SK, Harris D, et al. Phenylarsine oxide blocks interleukin-1β-induced activation of the nuclear transcription factor NF-κB, inhibits proliferation, and induces apoptosis of acute myelogenous leukemia cells. Blood. 1999; 94:2844–2853. PMID: 10515888.
Article34. Barkett M, Xue D, Horvitz HR, Gilmore TD. Phosphorylation of IκB-α inhibits its cleavage by caspase CPP32 in vitro. J Biol Chem. 1997; 272:29419–29422. PMID: 9367996.35. Korsmeyer SJ. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992; 80:879–886. PMID: 1498330.
Article36. Li L, Aggarwal BB, Shishodia S, Abbruzzese J, Kurzrock R. Nuclear factor-κB and IκB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer. 2004; 101:2351–2362. PMID: 15476283.
Article37. Liptay S, Weber CK, Ludwig L, Wagner M, Adler G, Schmid RM. Mitogenic and antiapoptotic role of constitutive NF-κB/Rel activity in pancreatic cancer. Int J Cancer. 2003; 105:735–746. PMID: 12767057.
Article38. Kong R, Sun B, Jiang H, et al. Downregulation of nuclear factor-κB p65 subunit by small interfering RNA synergizes with gemcitabine to inhibit the growth of pancreatic cancer. Cancer Lett. 2010; 291:90–98. PMID: 19880242.
Article39. Lee JU, Hosotani R, Wada M, et al. Role of Bcl-2 family proteins (Bax, Bcl-2 and Bcl-X) on cellular susceptibility to radiation in pancreatic cancer cells. Eur J Cancer. 1999; 35:1374–1380. PMID: 10658530.
Article40. Lee MA, Park GS, Lee HJ, et al. Survivin expression and its clinical significance in pancreatic cancer. BMC Cancer. 2005; 5:127. PMID: 16202147.
Article41. Tamm I, Kornblau SM, Segall H, et al. Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin Cancer Res. 2000; 6:1796–1803. PMID: 10815900.42. Bai J, Sui J, Demirjian A, Vollmer CM Jr, Marasco W, Callery MP. Predominant Bcl-XL knockdown disables antiapoptotic mechanisms: tumor necrosis factor-related apoptosis-inducing ligand-based triple chemotherapy overcomes chemoresistance in pancreatic cancer cells in vitro. Cancer Res. 2005; 65:2344–2352. PMID: 15781649.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cucurbitacin E’s Anti-Cancer Effects on HCT116 Human Colon Cancer Cells by Controlling Expression and Phosphorylation Levels of Caspase-9, eIF-2α, and ATF-4
- NF-kappaB-Mediated Regulation of Osteoclastogenesis
- Inhibition of Wnt Signaling by Silymarin in Human Colorectal Cancer Cells
- The edible ethanol extract of Rosa hybrida suppresses colon cancer progression by inhibiting the proliferation-cell signaling-metastasis axis
- Kahweol from Coffee Induces Apoptosis by Upregulating Activating Transcription Factor 3 in Human Colorectal Cancer Cells