Korean J Physiol Pharmacol.
2018 May;22(3):311-319. 10.4196/kjpp.2018.22.3.311.
Nobiletin attenuates neurotoxic mitochondrial calcium overload through K⺠influx and ΔΨ(m) across mitochondrial inner membrane
- Affiliations
-
- 1Department of Physiology, Jeju National University, Jeju 63243, Korea. syeun@jejunu.ac.kr
- 2Department of Histology, Jeju National University, Jeju 63243, Korea.
- 3Department of Biology, Jeju National University, Jeju 63243, Korea.
- 4College of Applied Life Science SARI, Jeju National University, Jeju 63243, Korea.
- 5Department of Anatomy, College of Medicine, Korea University, Seoul 02841, Korea.
- 6Institute of Medical Science, Jeju National University, Jeju 63243, Korea.
- KMID: 2410095
- DOI: http://doi.org/10.4196/kjpp.2018.22.3.311
Abstract
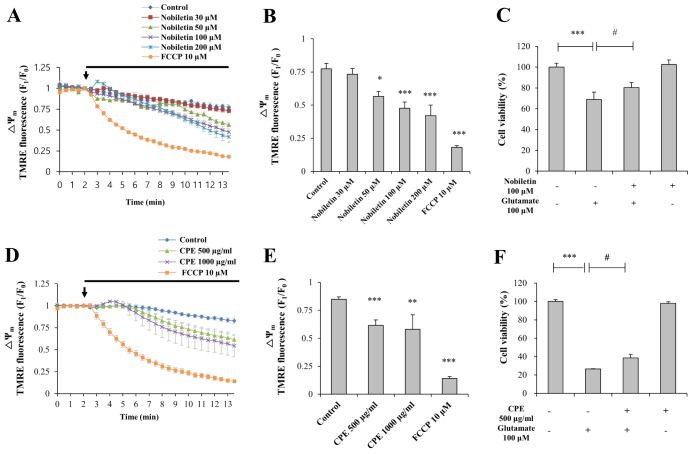
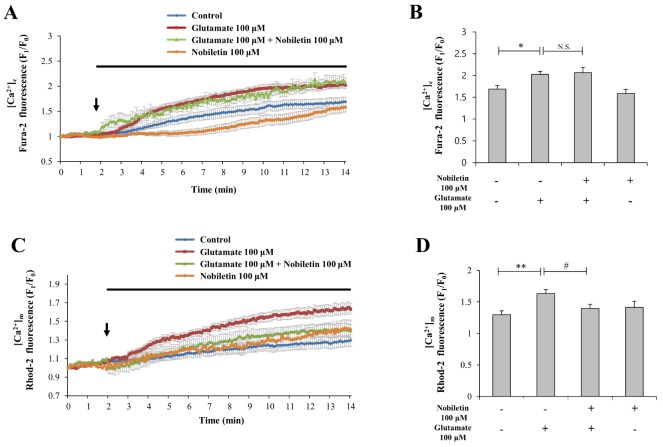
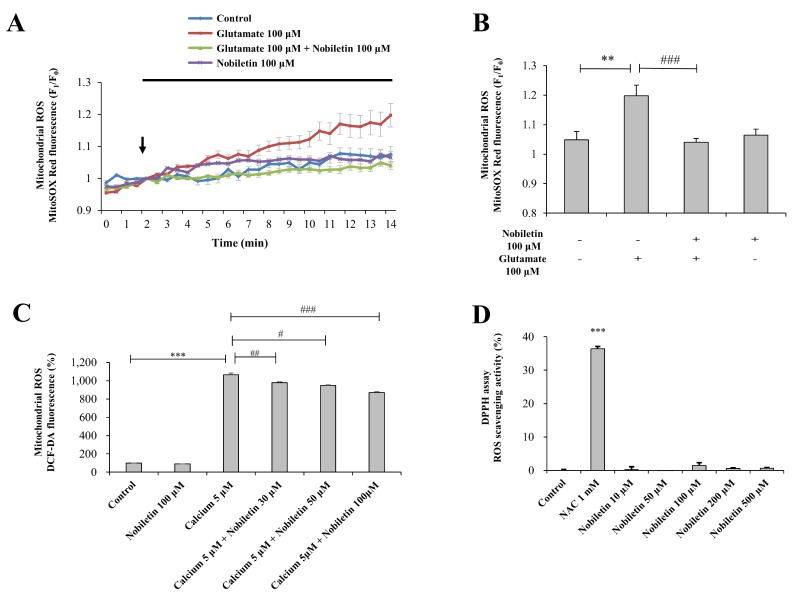
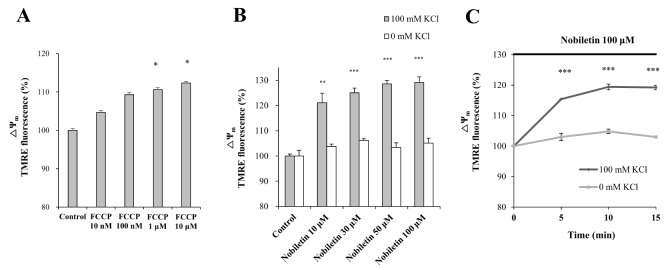
- Mitochondrial calcium overload is a crucial event in determining the fate of neuronal cell survival and death, implicated in pathogenesis of neurodegenerative diseases. One of the driving forces of calcium influx into mitochondria is mitochondria membrane potential (ΔΨ(m)). Therefore, pharmacological manipulation of ΔΨ(m) can be a promising strategy to prevent neuronal cell death against brain insults. Based on these issues, we investigated here whether nobiletin, a Citrus polymethoxylated flavone, prevents neurotoxic neuronal calcium overload and cell death via regulating basal ΔΨ(m) against neuronal insult in primary cortical neurons and pure brain mitochondria isolated from rat cortices. Results demonstrated that nobiletin treatment significantly increased cell viability against glutamate toxicity (100 µM, 20 min) in primary cortical neurons. Real-time imaging-based fluorometry data reveal that nobiletin evokes partial mitochondrial depolarization in these neurons. Nobiletin markedly attenuated mitochondrial calcium overload and reactive oxygen species (ROS) generation in glutamate (100 µM)-stimulated cortical neurons and isolated pure mitochondria exposed to high concentration of Ca²âº (5 µM). Nobiletin-induced partial mitochondrial depolarization in intact neurons was confirmed in isolated brain mitochondria using a fluorescence microplate reader. Nobiletin effects on basal ΔΨ(m) were completely abolished in Kâº-free medium on pure isolated mitochondria. Taken together, results demonstrate that K⺠influx into mitochondria is critically involved in partial mitochondrial depolarization-related neuroprotective effect of nobiletin. Nobiletin-induced mitochondrial K⺠influx is probably mediated, at least in part, by activation of mitochondrial K⺠channels. However, further detailed studies should be conducted to determine exact molecular targets of nobiletin in mitochondria.
Keyword
MeSH Terms
-
Animals
Brain
Calcium*
Cell Death
Cell Survival
Citrus
Fluorescence
Fluorometry
Glutamic Acid
Membrane Potential, Mitochondrial
Membrane Potentials
Membranes*
Mitochondria
Neurodegenerative Diseases
Neurons
Neuroprotective Agents
Rats
Reactive Oxygen Species
Calcium
Glutamic Acid
Neuroprotective Agents
Reactive Oxygen Species
Figure
Reference
-
1. Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007; 87:99–163. PMID: 17237344.
Article2. Duchen MR. Mitochondria, calcium-dependent neuronal death and neurodegenerative disease. Pflugers Arch. 2012; 464:111–121. PMID: 22615071.
Article3. Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990; 258:C755–C786. PMID: 2185657.
Article4. Ishida H, Hirota Y, Genka C, Nakazawa H, Nakaya H, Sato T. Opening of mitochondrial K(ATP) channels attenuates the ouabaininduced calcium overload in mitochondria. Circ Res. 2001; 89:856–858. PMID: 11701611.5. Sanz-Blasco S, Valero RA, Rodríguez-Crespo I, Villalobos C, Núñez L. Mitochondrial Ca2+ overload underlies Ab oligomers neurotoxicity providing an unexpected mechanism of neuroprotection by NSAIDs. PLoS One. 2008; 3:e2718. PMID: 18648507.6. Valero RA, Senovilla L, Núñez L, Villalobos C. The role of mitochondrial potential in control of calcium signals involved in cell proliferation. Cell Calcium. 2008; 44:259–269. PMID: 18241916.
Article7. Wu JJ, Cui Y, Yang YS, Jung SC, Hyun JW, Maeng YH, Park DB, Lee SR, Kim SJ, Eun SY. Mild mitochondrial depolarization is involved in a neuroprotective mechanism of citrus sunki peel extract. Phytother Res. 2013; 27:564–571. PMID: 22678994.8. Yamamoto Y, Shioda N, Han F, Moriguchi S, Nakajima A, Yokosuka A, Mimaki Y, Sashida Y, Yamakuni T, Ohizumi Y, Fukunaga K. Nobiletin improves brain ischemia-induced learning and memory deficits through stimulation of CaMKII and CREB phosphorylation. Brain Res. 2009; 1295:218–229. PMID: 19646972.
Article9. Yabuki Y, Ohizumi Y, Yokosuka A, Mimaki Y, Fukunaga K. Nobiletin treatment improves motor and cognitive deficits seen in MPTPinduced Parkinson model mice. Neuroscience. 2014; 259:126–141. PMID: 24316474.
Article10. Onozuka H, Nakajima A, Matsuzaki K, Shin RW, Ogino K, Saigusa D, Tetsu N, Yokosuka A, Sashida Y, Mimaki Y, Yamakuni T, Ohizumi Y. Nobiletin, a citrus flavonoid, improves memory impairment and Ab pathology in a transgenic mouse model of Alzheimer's disease. J Pharmacol Exp Ther. 2008; 326:739–744. PMID: 18544674.11. Nagase H, Omae N, Omori A, Nakagawasai O, Tadano T, Yokosuka A, Sashida Y, Mimaki Y, Yamakuni T, Ohizumi Y. Nobiletin and its related flavonoids with CRE-dependent transcription-stimulating and neuritegenic activities. Biochem Biophys Res Commun. 2005; 337:1330–1336. PMID: 16253614.
Article12. Cui Y, Wu J, Jung SC, Park DB, Maeng YH, Hong JY, Kim SJ, Lee SR, Kim SJ, Kim SJ, Eun SY. Anti-neuroinflammatory activity of nobiletin on suppression of microglial activation. Biol Pharm Bull. 2010; 33:1814–1821. PMID: 21048305.
Article13. Choi SY, Hwang JH, Ko HC, Park JG, Kim SJ. Nobiletin from citrus fruit peel inhibits the DNA-binding activity of NF-kappaB and ROS production in LPS-activated RAW 264.7 cells. J Ethnopharmacol. 2007; 113:149–155. PMID: 17611060.14. Eun SY, Jung SJ, Park YK, Kwak J, Kim SJ, Kim J. Effects of capsaicin on Ca2+ release from the intracellular Ca2+ stores in the dorsal root ganglion cells of adult rats. Biochem Biophys Res Commun. 2001; 285:1114–1120. PMID: 11478769.15. Scaduto RC Jr, Grotyohann LW. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J. 1999; 76:469–477. PMID: 9876159.
Article16. Iglesias-González J, Sánchez-Iglesias S, Beiras-Iglesias A, Soto-Otero R, Méndez-Álvarez E. A simple method for isolating rat brain mitochondria with high metabolic activity: effects of EDTA and EGTA. J Neurosci Methods. 2013; 213:39–42. PMID: 23261657.
Article17. Blattner JR, He L, Lemasters JJ. Screening assays for the mitochondrial permeability transition using a fluorescence multiwell plate reader. Anal Biochem. 2001; 295:220–226. PMID: 11488625.
Article18. Cho B, Cho HM, Jo Y, Kim HD, Song M, Moon C, Kim H, Kim K, Sesaki H, Rhyu IJ, Kim H, Sun W. Constriction of the mitochondrial inner compartment is a priming event for mitochondrial division. Nat Commun. 2017; 8:15754. PMID: 28598422.
Article19. Cui Y, Park JY, Wu J, Lee JH, Yang YS, Kang MS, Jung SC, Park JM, Yoo ES, Kim SH, Ahn Jo S, Suk K, Eun SY. Dieckol attenuates microglia-mediated neuronal cell death via ERK, Akt and NADPH oxidase-mediated pathways. Korean J Physiol Pharmacol. 2015; 19:219–228. PMID: 25954126.
Article20. Peng TI, Jou MJ. Oxidative stress caused by mitochondrial calcium overload. Ann N Y Acad Sci. 2010; 1201:183–188. PMID: 20649555.
Article21. Feissner RF, Skalska J, Gaum WE, Sheu SS. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front Biosci (Landmark Ed). 2009; 14:1197–1218. PMID: 19273125.22. Szewczyk A, Jarmuszkiewicz W, Kunz WS. Mitochondrial potassium channels. IUBMB Life. 2009; 61:134–143. PMID: 19165895.
Article23. Testai L, Martelli A, Marino A, D'Antongiovanni V, Ciregia F, Giusti L, Lucacchini A, Chericoni S, Breschi MC, Calderone V. The activation of mitochondrial BK potassium channels contributes to the protective effects of naringenin against myocardial ischemia/ reperfusion injury. Biochem Pharmacol. 2013; 85:1634–1643. PMID: 23567997.24. Saponara S, Testai L, Iozzi D, Martinotti E, Martelli A, Chericoni S, Sgaragli G, Fusi F, Calderone V. (+/-)-Naringenin as large conductance Ca2+-activated K+ (BKCa) channel opener in vascular smooth muscle cells. Br J Pharmacol. 2006; 149:1013–1021. PMID: 17088866.25. Bednarczyk P, Kicinska A, Jarmuszkiewicz W, Debowska R, Szewczyk A. Flavonoids as natural modulators of mitochondrial potassium channel. Biophys J. 2017; 112:405a–406a.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nobiletin Exhibits Neuroprotective Effects against Mitochondrial Complex I Inhibition via Regulating Apoptotic Signaling
- 4-hydroxy-2(E)-Nonenal facilitates NMDA-Induced Neurotoxicity via Triggering Mitochondrial Permeability Transition Pore Opening and Mitochondrial Calcium Overload
- Restoring mitochondrial dynamics in neuronal health through photobiomodulation
- Mitochondrial calcium uniporter inhibition attenuates mouse bone marrow-derived mast cell degranulation induced by beta-1,3-glucan
- A Novel Nicotinamide Adenine Dinucleotide Correction Method for Mitochondrial Ca2+ Measurement with FURA-2-FF in Single Permeabilized Ventricular Myocytes of Rat